Proceedings of the 35th Meeting
Working Group on Prolamin Analysis
and Toxicity (PWG)
Edited by Peter Koehler
May 2022
Preface
The 35th Meeting of the Working Group on Prolamin Analysis and Toxicity (PWG) was held online via Zoom on 31st March and 1st April 2022. Initially, the meeting was planned for autumn 2021as a physical meeting in Wageningen, The Netherlands. Due to the global Covid 19 pandemic it was postponed to the End of March 2022 and, finally, the unclear situation brought me to the decision to do it as an online meeting and to host it by myself. To enable participation of persons from overseas the meeting was held on two days in the afternoon, respectively. Due to the flexibility of the initial host René Smulders it was possible to cancel all preliminary arrangements in Wageningen and switch to the online format. 78 Persons registered for the meeting and on average 55 participants were online during the meeting. Apart from most of the group members, the audience comprised invited speakers, guests from academia, industry, and international coeliac societies. Representatives from cereal starch producers, producers of gluten-free foods, as well as manufacturers of kits for gluten analysis participated from industry. Despite the online format, the audience was very active in discussing the presentations. Analytical and clinical work in the field of coeliac disease (CD), gluten intolerances, gluten and wheat breeding done not only in the labs of PWG members were presented in eight talks. The symposium “Gluten analysis and clinical effects of low gluten doses” comprised two presentations of PWG members and one from a coeliac society. In the symposium, the analytical and also the clinical issues of low gluten uptake were highlighted. As usual, one presentation that focussed on regulatory aspects of gluten analysis and labelling concluded the meeting. After the sessions, there was enough time for discussions. I would like to express my thanks to all speakers for their excellent presentations and to all participants for their active contributions to the meeting. I am in particular grateful to Technical University of Munich for providing the Zoom platform for the meeting. No technical issues occurred during the meeting. This made the meeting a success despite the general restrictions due to the online format. Finally, I express my gratitude to all friends, colleagues, sponsors and participants for their inspiration and ongoing support of the PWG and the meeting.
Esslingen, May 2022 Peter Koehler
Executive Summary
Twelve presentations covered aspects related to gluten, coeliac disease (CD) and other hypersensitivities, wheat protein digestibility as well as legal issues. All authors provided abstracts that are compiled in this proceedings book.
Analytical session
Four presentations were given in this session. One presentation covered the influence of processing such as breadmaking on the extractability and proteomic analysis of gluten proteins. Advanced proteomics work was shown in this talk. Two contributions were on gluten analysis by ELISA, one described the application of the R5 sandwich ELISA to a broad range of different foods and the other introduced a novel direct ELISA based on the R5 antibody for the quantitation of partially hydrolysed gluten in fermented foods (e.g., beer) with a low limit of quantitation (LOQ). The last presentation in the analytical session elucidated the mechanism of modification of gluten peptides by tissue transglutaminase (TG2) as part of the pathomechanism of CD.
Clinical session
This session included four presentations. The first study aimed at identifying phenotypic traits of wheat linked to proteolysis during bread in-vitro digestion. There was a genotypic effect that influenced proteolysis during digestion showing that the cultivar might affect digestibility. The second talk described the detoxifying capabilities of the peptidase E40 on residual gluten immunogenic peptides generated after gastrointestinal digestion. E40 appears to be a promising candidate for the oral enzymatic therapy of gluten intolerance in CD. The third study aimed at elucidating the reasons for lack of improvement in coeliac patients on a gluten-free diet and suggests a low FODMAP approach as a promising tool for helping those patients. The last presentation of this session was on the clinical overlap of type-1 diabetes (T1D) and CD. Apparently, the mucosa of T1D patients seems to be more sensitive to the pro-inflammatory effects of gliadin and its peptides P31-43 and might contribute to the baseline intestinal inflammation and to the susceptibility of T1D patients to develop concomitant CD.
Symposium: Gluten analysis and clinical effects of low gluten doses
Analysis of partially hydrolysed gluten is still a challenge and poses critical questions to the CD community: What is the best reference material for partially hydrolysed gluten? Do competitive ELISAs miss CD-active peptides? Do CD-active peptides detected by LC-MS/MS pose a risk for CD patients? The second talk concluded that there is reason to be worried over the finding that even very well treated coeliac disease patients may have low level inflammation in their mucosa due to low gluten uptake. Finally, the last presentation given by Coeliac UK made clear that there is a misunderstanding of the statement ‘gluten free’ among the UK coeliac community. Also, there is interest in alternative therapeutics for CD but the coeliac community needs to be better informed about what is involved in clinical trials.
Analytical research reports
Proteomics and metabolomics analysis of flours, doughs and breads from wheat, emmer and spelt and using yeast and sourdough fermentation processes
Antoine H.P. America* , Peter R. Shewry2 , Alison Lovegrove2 , Jane L. Ward2 , Petra Kuiper3 , Jan Philip van Straaten3 , Daisy Jonkers4 , Fred Brouns5
1 Plant Sciences Group, Wageningen University and Research, Wageningen, The Netherlands 2 Rothamsted Research, Harpenden, United Kingdom 3 Dutch Bakery Center, Wageningen, The Netherlands 4 Division of Gastroenterology-Hepatology, Maastricht University, The Netherlands 5 Department of Human Biology and School for Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, The Netherlands
Abstract
In the Well-on-Wheat project we study the effects of either wheat source, fermentation process as well as baking process on the global composition of the baked product and aim to relate this to potential effects in the gut during digestion. We performed a highly detailed molecular analysis of the metabolite, fibre and protein composition of the different stages during breadmaking (https://doi.org/10.1016/j.foodchem.2021.131710). Wholemeal flours from blends of bread wheat, emmer and spelt were processed into bread using yeast-based and sourdough fermentation. Metabolites were analysed by NMR and GC-MS, fibres by HPLC and enzyme assays, and proteome composition was profiled by LC-MS analysis. The total dietary fibre and fructans content was significantly higher in wheat than the spelt and emmer flours. Breadmaking using either sourdough or yeast resulted in many changes in composition from flour to dough to bread including increases in organic acids and mannitol in the sourdough system and increases in amino acids and sugars (released by hydrolysis of proteins and starch, respectively) in both processing systems. Fructans and raffinose (the major endogenous FODMAPs) were reduced more by yeast than sourdough fermentation. Proteins were extracted by two different extraction protocols displaying remarkable differences in the selective extractability of a subset of proteins contrasting sourdough versus yeast fermented bread. Remarkably, after baking a major loss of protein, extracted in 50% isopropanol, was observed in the yeast fermented bread, while in sourdough fermented bread this reduction was less severe. Addition of reducing agent (DTT) to the extraction buffer could only partially recover the nonextracted proteins. We hypothesize that in sourdough fermentation a more reducing environment protects proteins from forming oxidised crosslinks during the baking process. Multivariate analysis of the peptide/protein profiles quantified by LC-MS displayed different responses of several classes of proteins like gliadins, glutenins, globulins and amylase/protease inhibitors as result of either yeast or sourdough fermentation and baking.
Loaves produced from the above detailed bread products are currently used in a food intervention study to investigate any potential effects relating to irritable bowel syndrome.
Collaborative study using a wide range of matrices for gluten analysis by R5 sandwich ELISA RIDASCREEN® Gliadin
Tina Dubois1 , Lisa Zimmermann2 , Markus Lacorn1 , Teresa-Maria Schinabeck2 , Simone Loos-Theisen2 , Thomas Weiss1 , Katharina Scherf3
1 R-Biopharm AG, An der neuen Bergstraße 17, 64297 Darmstadt, Germany 2 Hochschule Geisenheim University, Department of Food Safety, Von-LadeStraße 1, 65366 Geisenheim, Germany 3 Karlsruhe Institute of Technology (KIT), Institute of Applied Biosciences, Department of Bioactive and Functional Food Chemistry, Karlsruhe, Germany
Abstract
According to Codex Alimentarius, food products containing less than 20 mg/kg gluten can be labeled as “gluten-free.” Since 2002, the R5 antibody method allowed determination of gluten levels and led to a huge improvement of products available to celiac disease patients.
The R5-containing test kit RIDASCREEN® Gliadin R7001 in combination with the cocktail solution was endorsed as Codex Type 1 Method in 2006 based on a collaborative study with corn-based bread, rice-based dough, wheat starches, rice, and corn flour. In 2012, the method was approved as First Action Official MethodSM 2012.01 with an “in foods” claim. For Final Action in 2016, the matrix claim was reduced to rice- and corn-based matrixes. This was solely a formal decision by AOAC as it is general AOAC policy that only the matrices verified in a collaborative study can be claimed. It did not convey any information about the possible applicability to other matrices. Nevertheless, the reduced scope of the method led to severe irritations in the analytical community.
Therefore, R-Biopharm decided to start a new collaborative study to demonstrate the wide applicability of Official Method 2012.01 for the quantitative analysis of gliadin in soy, starches, pseudo cereals, legumes, spices, juice, nut nougat crème, cream cheese, pesto, meat, vegetarian meat alternative, cookies, dessert, cake, fish, bread, candies, and potatoes. Materials for incurring were the MoniQA wheat flour and the PWG gliadin preparation. The gluten containing materials were added to the matrices prior to the main processing steps, which ranged from simple blending to cooking and frying up to baking at 180 °C for 1 h. Gliadin levels of the analysed samples ranged from 3.4 up to 27.4 mg gliadin per kg. All materials were prepared independently at the Hochschule Geisenheim University.
The results of the collaborative study with 14 participating laboratories showed recoveries ranging from 80 to 130%. Relative reproducibility standard deviations for contaminated samples were between 9.8 and 27.7%. Recoveries and standard deviations were independent of the processing of the different materials. The collaborative study results confirmed that the method is accurate and suitable to measure gliadin in important gluten-free food matrices. The title and applicability statement of Official Method 2012.01 were changed as proposed. All results were published in Journal AOAC: https://doi.org/10.1093/jaoacint/qsab148.
Direct R5 ELISA method for hydrolyzed samples: SENSISpec INgezim Hydrolyzed Gluten
Cristina Romero, Ángel Venteo, Isabel González
Eurofins Ingenasa, Madrid, Spain
Abstract
It is widely known that the quantification of gluten in hydrolysed samples means a challenge for any analytical technique. In fact, it is proven that the reference method for gluten detection (ELISA R5 sandwich) does not obtain the adequate recovery values in this kind of samples.
Different approaches have been developed to solve this issue (e.g., competitive ELISA). But now, new methods are available in the market, specifically designed to detect the hydrolysed gluten in a reliable and accurate manner.
This is the case of the SENSISpec INgezim Hydrolyzed Gluten R5, a direct ELISA capable to detect both, hydrolyzed and non-hydrolyzed gluten proteins. The method is based in chemically activated plate wells, where the gluten proteins can bind whether they are hydrolyzed or not. This way, the posterior addition of the correspondent conjugated R5 monoclonal antibody, provides the adequate recovery yields.
The total assay time ranges between 1 hour (for liquid samples), to 1 hour 40 minutes (for solid samples). The Sensitivity of the method is 0.25 ppm of Gluten, one of the lowest in the market.
The assay has been validated with real beer samples, obtaining a very high recovery values when comparing the sandwich ELISA
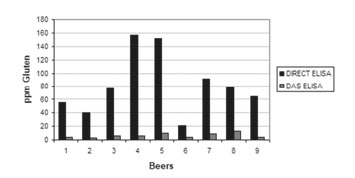
In addition, the method was compared and validated by the Spanish reference laboratory for gluten (Unit of Gluten from the National Centre of Biotechnology), obtaining equivalent results between the direct and the competitive ELISA. Concluding, the SENSISpec INgezim Hydrolyzed Gluten R5 is a perfect alternative for the allergens risk assessment in hydrolyzed matrices.
Identification of isopeptides between human TG2 and gluten peptides from wheat, rye, and barley
Barbara Lexhaller1 , Christina Ludwig2 , Katharina Scherf1,3
1 Karlsruhe Institute of Technology (KIT), Institute of Applied Biosciences, Department of Bioactive and Functional Food Chemistry, Karlsruhe, Germany 2 Bavarian Center for Biomolecular Mass Spectrometry (BayBioMS), Technical University of Munich, Freising, Germany 3 Leibniz-Institute for Food Systems Biology at the Technical University of Munich, Freising, Germany
Abstract
The intestinal tissue transglutaminase (TG2) plays a key role in the complex pathogenesis of Coeliac disease (CD). This Ca2+-dependent protein-glutamine γ-glutamyltransferase forms covalently linked complexes with gluten peptides, which induce the formation of antibodies against these complexes. The binding sites of the gluten peptide-TG2 complexes consist of intermolecular Nε -(γ-glutamyl)-lysine bonds, so-called isopeptides.
The aim of this study was subdivided into two parts: 1) the characterization of the gluten protein types and 2) the identification of the isopeptides between TG2 and gluten peptides from wheat, rye and barley by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Flour of wheat, rye and barley were isolated via Osborne fractionation and preparative HPLC for the characterization of the gluten protein types. These gluten protein types were then characterized by different analytical methods. In the second part of the study, a comprehensive detection of isopeptides was conducted. A proteomic workflow was developed to identify isopeptides based on discovery driven as well as targeted proteomic data using the software tools MaxQuant and Skyline. Further, the development of a two-component model system with human TG2 and gluten proteins laid the basis for the analysis of biologically formed isopeptides between gluten peptides and TG2. Gastrointestinal digestion of the gluten proteins was simulated by peptic, tryptic and chymotryptic hydrolysis to obtain gluten peptides. To mimic the subsequent modifications of the gluten peptides by TG2, the gluten peptides were incubated with human TG2 in a second model system simulating biological conditions. During this reaction, the gluten peptide-TG2 complexes were formed via isopeptide bonds. Subsequently, these complexes were hydrolysed and the resulting peptides and isopeptides were analysed by untargeted as well as targeted LC-MS/MS methods.
A total of 29 isopeptides were identified in 14 biological hydrolysates of gluten protein types of wheat, rye and barley incubated with TG2. The exact localization of the binding sites within the gluten peptides were already successfully determined by the non-targeted LC-MS/MS method for 18 isopeptides. These binding sites were reconfirmed by Parallel Reaction Monitoring (PRM) analysis, and, in addition, nine more binding sites were accurately determined by this analytical technique. Some of the identified isopeptides contain known CDspecific sequences, which are related to the immune response of CD.
5. Clinical research reports
How to breed wheat for improving bread proteins in vitro digestibility?
M. Lavoignat1,2, E. Bancel1 , M. Alric3 , S. Bagnon4 , M. Benigna4 , A. Chassin5 , S. Berges5 , S. Denis3 , A. Faye1 , E. Heumez6 , P.Y. Dymarski7 , L. Halupka7 , S. Perrochon1 , L. Rhazi8 , B. Valluis9 , F. Souply9 , M.C. Leroux9 , P. Giraudeau*, C. Ravel1
1 Université Clermont Auvergne-INRAE, UMR1095 GDEC, Clermont-Ferrand, France 2 AgroParisTech, Paris, France 3 Université Clermont Auvergne-INRAE, UMR454 MEDiS, Clermont-Ferrand, France 4 Qualtech, Vandoeuvre, France 5 UE INRAE PHACC, Clermont-Ferrand, France 6 UE INRAE GCIE, Estrées-Mons, France 7 Cerelab, Aiserey, France 8 UniLaSalle, Beauvais, France 9 ANMF, Paris, France * Representing UFS, Paris, France
Abstract
Grain proteins of wheat (Triticum aestivum) are composed of functional proteins and storage proteins, gliadins and glutenins. Gliadins and glutenins form the gluten polymeric network conferring dough rheological properties. Therefore, their content and composition strongly influence the aptitude for processing of wheat cultivars. Wheat is a staple food for human and mainly consumed as bread, after milling grains into flour. However, wheat grain proteins are partially resistant to gastrointestinal enzymes and are associated to several health issues related to gluten.
In this context, our study aims at identifying phenotypic traits linked to proteolysis during bread in-vitro digestion. Grains from 17 old and modern cultivars grown at two locations were phenotyped. Grain hardness, thousand-kernel-weight and grain nitrogen content were measured. Protein composition was determined by Reverse-Phase High Performance Liquid Chromatography (RP-HPLC). Flour polymers were characterized by Asymmetric Flow Field Flow Fractionation (AF4). Dough and gluten technological properties were evaluated by a Chopin Alveograph and a Glutomatic system, respectively. For each cultivar, breads were baked according to a standardized method and digested in vitro with a dynamic gastrointestinal system TIM-1. After two hours of digestion, a nitrogen balance was performed on samples from the stomach compartment, the small intestine and the ileal effluents (undigested fraction evacuated towards the colon) allowing to evaluate the quantity of nitrogen in the digested, ongoing digestion and non-digested fractions. A synthetic variable reflecting proteolysis at two hours of digestion (ratio of nitrogen in the digested compartment out of total nitrogen measured from all TIM-1compartments) was calculated and multivariate analyses were conducted.
Genotypic effect significantly influenced proteolysis at 2 h of digestion (ANOVA, pvalue = 0.013) which varied from 0.44 to 0.61 among varieties. However, bread proteins hydrolysis neither depended on the growing environment nor on the group of age of the cultivar. Proteolysis appeared to be related to some phenotypic traits such as grain protein content and composition. Identifying plant traits linked to proteolysis could help breeders improving this complex trait.
E40-glutenase efficiently detoxifies residual gluten immunogenic peptides in gastrointestinal digesta of soft and durum wheat-based food matrices
Gianfranco Mamone1 , Maria Cristina Comelli2 , Serena Vitale3 , Luigia Di Stasio1 , Katharina Kessler2 , Ilaria Mottola3 , Francesco Siano1 , Linda Cavaletti4 , Carmen Gianfrani3
1 Institute of Food Science, National Research Council of Italy, Via Roma 64, 83100 Avellino, Italy 2 Nemysis Limited, Suite 4.01 Ormond Building, 31-36 Ormond Quay Upper, Arran Quay, D07 F6DC Dublin, Ireland 3 Institute of Biochemistry and Cell Biology, Department of Biomedical Sciences, National Research Council of Italy, Via Pietro Castellino 111, 80131 Naples, Italy 4 Fondazione Istituto Insubrico Ricerca per la Vita, Via Roberto Lepetit 34, 21040 Gerenzano-Varese, Italy
Abstract
The high content of glutamine and proline amino acids makes the gluten proteins resistant to human digestive process, including gastric, duodenal and brush border membrane proteases. This prominent gastrointestinal resistance of gluten proteins ensures the survival in the gut lumen of large immunogenic peptides (GIPs) that stimulate inflammatory responses in patients with Celiac Disease (CeD). Over the last years, there has been a rising interest in developing novel therapeutic strategies aimed to detoxify the dietary gluten and, consequently, to improve CeD patient quality of life. We have previously demonstrated that the Endopeptidase-40 (E40) efficiently hydrolyzed gliadin proteins and the most immunogenic -gliadin 33-mer peptide, making them inactive to stimulate pro-inflammatory intestinal T cells in celiac patients (Cavaletti et al., Sci Rep 9, 13147 (2019); https://doi.org/10.1038/s41598-019-48299-7).
Herein, we comprehensively assessed the detoxifying capabilities of E40 on residual GIPs generated after the gastrointestinal digesta of liquid and solid wheat food matrices, as beer, bread and pasta. The Infogest protocols were applied to simulate human gastrointestinal digestion. Proteomic (LC-MS/MS) and immunological (R5-ELISA and celiac intestinal T cells) assays were used to compare the pattern of residual GIPs after gastric and gastrointestinal digestion of wheat-matrices treated with E40, added to the gastric phase.
Residual gluten content in E40-treated gastro-intestinal digesta assessed by R5 was found equal to, or less, the gluten-free threshold of 20 ppm, for both liquid and solid wheat-matrices. LCMS/MS analysis of E40-digestested samples revealed a dose-dependent degradation of whole gluten and GIPs, that reached more than 95% detoxification up to an enzyme:protein ratio of 1:50. Unlike in untreated, in E40-digesta none of the immunodominant-gliadin peptides survived, whilst residual - and -gliadin peptides were unable to stimulate celiac gut T cells. Overall, this study confirms that E40 is a promising candidate for the oral enzymatic therapy of gluten intolerance in CeD as a stand-alone enzyme, favoring the whole digestive process of gluten.
Treated coeliac patients with persistent gastrointestinal symptoms
Frida van Megen1,2, Gry Skodje3 , Marit B. Veierød4 , Christine Henriksen5 , Knut E. A. Lundin2,6
1 Department of Clinical services, Oslo University Hospital Rikshospitalet, Oslo, Norway 2 KG Jebsen Coeliac Disease Research Centre, University of Oslo, Oslo, Norway 3 Healthy Life Centre, Municipality of Nes, 2150 Årnes, Norway 4 Oslo Centre for Biostatistics and Epidemiology, Department of Biostatistics, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway 5 Department of Nutrition, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway 6 Department of Gastroenterology, Oslo University Hospital Rikshospitalet, Oslo, Norway
Abstract
Introduction: The treatment goals in coeliac disease include improvement of abdominal problem (similar to irritable bowel syndrome IBS), fatigue and reduced quality of life. Lack of improvement is frequent and due to non-compliance or persistent IBS-like symptoms [1].
Methods: We first investigated the magnitude of the problem with an internet-based survey [2]. Respondent in this survey were invited to a randomised controlled trial (RCT), if they had persistent symptoms and showed strict compliance with the gluten free diet (GFD) as measured with serology, duodenal biopsy and clinical interview [3]. In the RCT, they were randomised 1:1 to either continue their regular GFD, or to moderately reduce the intake of fermentable oligo-, di-, monosaccharides and polyols (FODMAP) for four weeks ]4].
Results: The internet survey was responded by 3834 anonymous participants that had biopsyproven coeliac disease and a GFD for at least 12 months [5]. Approximately 1/3 had a Celiac Disease Index of 45 or more, indicating relatively poor quality of life. From this cohort, individuals with high symptom burden were invited to the second phase [4]. Seventy patients entered the randomized control trial, all of them had mucosal healing in duodenal biopsies. A clinical assessment, a validated adherence tool (Celiac Adherence Test [6]) and gluten peptides in urine and stool [7] was performed [8]. We did find some cases of gluten peptides in stool, possibly reflecting minor transgression. In the RCT we instructed to the patients to adhere to a “moderately low FODMAP diet” as part of their GFD. This significantly reduced their symptoms as early as after 1 week. We will do a follow-up study this autumn.
Discussion: The low FODMAP approach is a promising tool for helping coeliac disease patients on a GFD but with persistent symptoms in our hands as well as in the hands of others [9,10]. The treatment is complicated and requires a dedicated clinical dietician expertise.
References
1. Al-Toma A, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019; 7(5): 583-613.
2. van Megen F, et al. High disease burden in treated celiac patients - a web-based survey. Scand J Gastroenterol 2021; 56(8): 882-888.
3. Skodje GI, et al. Detection of gluten immunogenic peptides and the Celiac Disease Adherence Test to monitor gluten-free diet: a pilot study. Eur J Clin Nutr 2022; 76(6): 902- 903.
4. van Megen F, et al. A Low FODMAP Diet Reduces Symptoms in Treated Celiac Patients With Ongoing Symptoms-A Randomized Controlled Trial. Clin Gastroenterol Hepatol 2022; in press. https://doi.org/10.1016/j.cgh.2022.01.011.
5. van Megen F, et al. High disease burden in treated celiac patients - a web-based survey. Scand J Gastroenterol 2021; 56(8): 882-888.
6. Leffler DA, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol 2009; 7(5): 530-536, 536 e1-2.
7. Moreno ML, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017; 66(2): 250-257.
8. Skodje GI, et al. Detection of gluten immunogenic peptides and the Celiac Disease Adherence Test to monitor gluten-free diet: a pilot study. Eur J Clin Nutr 2022; 76: 902- 903.
9. Roncoroni L, et al. A Low FODMAP Gluten-Free Diet Improves Functional Gastrointestinal Disorders and Overall Mental Health of Celiac Disease Patients: A Randomized Controlled Trial. Nutrients 2018; 10(8): 1023.
10. Trott N, et al. Adult celiac disease with persistent IBS-type symptoms: a pilot study of an adjuvant FODMAP diet. Gastroenterol Hepatol Bed Bench 2021; 14(4): 304-310.
Intestinal inflammation and type 1 diabetes: The role of gliadin peptides
V. Discepolo, M. Maglio, M. Nanayakkara, E. Mozzillo, A. Franzese, A. Marano, M. Boccia, R. Mandile, R. Auricchio, M.V. Barone, R. Troncone
Department of Translational Medical Sciences and European Laboratory for the Investigation of Food Induced Diseases (ELFID), University of Naples Federico II, Naples, Italy
Abstract
In the last decades, the global incidence of autoimmune diseases increased exponentially, especially in industrialized countries [1,2]. Among these diseases, type-1 diabetes (T1D) and celiac disease (CeD) show a clinical overlap and also share genetic and pathogenetic mechanisms. Environmental factors, such as intestinal viral infections, through an alteration of immune homeostasis, might contribute to trigger both diseases [3]. In a previous work4, we showed that pancreatic beta islet cells of T1D patients display increased levels of interleukin (IL)-15 and myxovirus resistance protein 1 (MxA), a downstream effector of the type1 IFN receptor, induced in response to viral infections, both markers are also increased in CeD patients’ gut.
In addition to infectious triggers, dietary factors play a role: evidences suggest that gluten promotes T1D onset in susceptible animal models (i.e. NOD mice BALB mice) [5-7], and T1D patients seem to be more sensitive to the pro-inflammatory effects of gliadin [8].
On this basis, we hypothesize that intestinal innate immune cytokines promoted in response to environmental triggers including viral infections and gliadin peptides that remain undigested in the intestinal lumen, may contribute to induce autoreactive T cells and autoimmune processes.
To address this hypothesis, we investigated the baseline inflammation and the impact of gliadin on the innate immune activation in the small intestine of patients with T1D. We enrolled 15 T1D patients in the absence of CeD or CeD specific autoantibodies, and 15 non-CeD non-T1D controls undergoing upper gastrointestinal endoscopy for clinical reasons. All patients enrolled displayed a normal intestinal architecture and negative CeD-specific autoantibodies. In the duodenal biopsies of those patients we studied the expression of IL-15 (a key innate immune cytokine in CeD pathogenesis), MxA (a proxy for the type-1 IFN pathway activation) and Ki67, that serves as a marker of proliferating enterocytes in the duodenal crypts.
At baseline, an increased expression of IL-15 was found in the surface epithelium (p<0.01), in the crypts (p<0.001) and in the lamina propria (p<0.01) of duodenal biopsies of T1D patients compared to controls. Increased expression of MxA was highlighted in the small intestine by IHC (p<0.01) and WB (p<0.05). An increase in proliferating crypts was also observed by IHC (p<0.001) at baseline and in response to gliadin (p<0.05) in organ cultures. Furthermore, we observed an increased expression of MxA (by WB) in duodenal organ cultures of T1D patients after overnight treatment with gliadin (p<0.05) and its peptide P31-43 (p<0.001).
In conclusion, our data indicate that T1D patients, even in the absence of CeD-specific antibodies or symptoms suggestive of an intestinal injury, show small intestinal activation of innate immune pathways (i.e. IL-15 and type-1 IFN) known to be upregulated in the duodenum of CeD patients, likely in response to dysbiosis and viral infections respectively; the same patients present increased crypts’ proliferation, likely also in response to higher IL-15 levels. This suggests that common environmental triggers (infections, dietary factors, dysbiosis) altering the intestinal immune homeostasis might contribute to enhance pro-inflammatory immune responses that ultimately lead to autoreactive T cells and promotion of both CeD and T1D. Moreover, the mucosa of T1D patients seems to be more sensitive to the pro-inflammatory effects of gliadin and its peptides P31-43, suggesting it might contribute to the baseline intestinal inflammation and to the susceptibility of T1D patients to develop concomitant CeD.
References
1. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989- 2013: a multicentre prospective registration study. Diabetologia, 2019; 62(3): 408-417.
2. King JA, Jeong J, Underwood FE, et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am J Gastroenterol 2020;115(4): 507-525.
3. Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol 2013;13(4): 294-302.
4. Chen J, Feigenbaum L, Awasthi P, et al. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Rα. Proc Natl Acad Sci USA 2013;110(33): 13534- 13539.
5. Hansen CH, Krych L, Buschard K, et al. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes 2014; 63(8): 2821-2832.
6. Marietta EV, Gomez AM, Yeoman C, et al. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS One 2013; 8(11): e78687.
7. Larsen J, Weile C, Antvorskov JC, et al. Effect of dietary gluten on dendritic cells and innate immune subsets in BALB/c and NOD mice. PLoS One 2015; 10(3): e0118618.
8. Auricchio R, Paparo F, Maglio M, et al. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes 2004; 53(7): 1680-1683.
Symposium: Gluten analysis and clinical effects of low gluten doses
Analysis of partially hydrolysed gluten in fermented foods
Katharina Scherf
Karlsruhe Institute of Technology (KIT), Institute of Applied Biosciences, Department of Bioactive and Functional Food Chemistry, Karlsruhe, Germany
Abstract
The three main challenges for gluten analysis include the complexity of gluten as an analyte, the different sensitivities of individual coeliac disease patients and the respective drawbacks of each analytical method such as polymerase chain reaction, enzyme-linked immunosorbent assay (ELISA) and liquid chromatography mass spectrometry (LC-MS/MS). Gluten proteins are storage proteins of wheat, rye and barley and their content and composition vary depending on botanical origin and environmental factors. Food processing may cause further changes, including heat-induced modifications or partial hydrolysis during fermentation.
Known challenges for ELISA testing are that the monoclonal antibodies (mAbs) employed in food analytical test kits recognize different gluten protein fractions (gliadins, glutenins, secalins, hordeins) with different specificity and sensitivity. Furthermore, epitope mapping showed that about 66% of CD-active peptides remain undetected, because the epitopes recognized by the ELISA mAbs do not react with all amino acid sequences present in CD-active peptides. These findings also show that ELISA mAbs do not necessarily reflect CD-activity in patients. The analysis of partially hydrolysed gluten is particularly challenging, because a competitive or direct ELISA test kit needs to be used. Although a competitive ELISA has been validated to be fit-for-purpose by international collaborative studies, the US Food and Drug Administration (FDA) does not accept the method. In their view, the peptic-tryptic prolamin hydrolysate that is used as the reference material for calibration does not adequately represent the gluten peptide mixture in different fermented foods, such as beers or malt extracts. Consequently, barley-based beers that have been rendered gluten-free through appropriate processing cannot bear a gluten-free claim in the US, because the FDA has the opinion that there is no scientifically proven method to quantitate partially hydrolysed gluten.
Comparative analyses of beer samples by competitive ELISA and LC-MS/MS resulted in 100 mg/kg and 400 mg/kg of gluten, respectively. Eight hordein peptides bearing known CDactive epitopes were detected by untargeted nanoLC-MS/MS analyses of barley-based glutenfree beers. One of these beers had detectable gluten (28.9 mg/kg, competitive ELISA) and it also contained 0.9 mg/kg of one CD-active peptide and very low levels of two more. Further work will focus on analysing more beer samples and providing further quantitative data.
Taken together, there are still some outstanding questions: i) What is the best reference material for partially hydrolysed gluten? ii) Do competitive ELISAs miss CD-active peptides? iii) Do CD-active peptides detected by LC-MS/MS pose a risk for CD patients? An international consortium of Prolamin Working Group members and associated partners will set out to find answers within the scope of the ImmunoSafe-CeD project, funded by the EU Joint Programming Initiative - A healthy diet for a healthy life.
Clinical effects of low-level gluten exposure – a critical view
Knut E. A. Lundin1,2
1 KG Jebsen Coeliac Disease Research Centre, University of Oslo, Oslo, Norway 2 Department of Gastroenterology, Oslo University Hospital Rikshospitalet, Oslo, Norway
Abstract
The treatment of coeliac remains the gluten free diet (GFD), but questions remain on how much gluten that can be tolerated and the consequences of dietary transgressions.
Introduction and methods: A literature search on Pubmed focused on consequences of lack of mucosal healing, national recommendations on level of gluten in food, challenge studies using defined gluten amounts and new methods to evaluate compliance.
Results and discussion: Mucosal healing in coeliac disease is important as persistant inflammation is associated with increased mortality [1]. However, the essential question remains; how much gluten is too much? Maybe no gluten at all?
There are few challenge studies that investigate the lowest dose able to induce mucosal pathology. The hallmark study by Catassi suggested that 50 mg could do this in children [2]. Other studies have typically used larger amounts [3]. Thus, careful titration challenges are largely lacking, and none look at hard endpoints.
A topic of particular interest is the use of wheat-starch based diet vs natural gluten-free diet. Many countries allow this in the GFD, whereas others prohibit any detectable gluten. This topic was the focus of research some years ago [4-7]. The common outcome of these studies was that the small amounts of gluten in wheat starch was not significant. However, today’s strategy for drug treatment of coeliac disease focus on drugs against the “background exposure” of gluten to celiacs [8]. And poor compliance, as evidenced by gluten peptides in urine or stool, is frequent [9,10]. There is reason to be worried over the finding that even very well treated coeliac disease patients may have low level inflammation in their mucosa [11]. There is still a lot to be learned about the optimal delivery of care to our coeliac disease patients.
References
1. Ludvigsson JF, et al. Small-intestinal histopathology and mortality risk in celiac disease. JAMA, 2009; 302(11): 1171-1178.
2. Catassi C, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr, 2007; 85(1): 160-166.
3. Lahdeaho ML, et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology, 2014; 146(7): 1649-1658.
4. Selby WS, et al. Persistent mucosal abnormalities in coeliac disease are not related to the ingestion of trace amounts of gluten. Scand J Gastroenterol, 1999; 34(9): 909-914.
5. Faulkner-Hogg KB, Selby WS, Loblay RH. Dietary analysis in symptomatic patients with coeliac disease on a gluten-free diet: the role of trace amounts of gluten and non-gluten food intolerances. Scand J Gastroenterol, 1999; 34(8): 784-789.
6. Collin P, et al. The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease? Aliment Pharmacol Ther, 2004; 19(12): 1277-1283.
7. Kaukinen K, et al. Wheat starch-containing gluten-free flour products in the treatment of coeliac disease and dermatitis herpetiformis. A long-term follow-up study. Scand J Gastroenterol, 1999; 34(2): 163-169.
8. Kivela L, et al. Current and emerging therapies for coeliac disease. Nat Rev Gastroenterol Hepatol, 2021; 18(3): 181-195.
9. Moreno ML, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut, 2017; 66(2): 250-257.
10. Stefanolo JP, et al. Real-World Gluten Exposure in Patients With Celiac Disease on GlutenFree Diets, Determined From Gliadin Immunogenic Peptides in Urine and Fecal Samples. Clin Gastroenterol Hepatol, 2021; 19(3): 484-491 e1.
11. Stamnaes J, et al. In Well-Treated Celiac Patients Low-Level Mucosal Inflammation Predicts Response to 14-day Gluten Challenge. Adv Sci (Weinh), 2021; 8(4): 2003526.
Everyday management of the gluten free diet
Heidi Urwin, Alice Andrews, Emily Hampton, Ruth Passmore.
Coeliac UK, 3rd Floor Apollo Centre, Desborough Road, High Wycombe, Bucks, HP11 2QW, United Kingdom
Abstract
The only current treatment for coeliac disease (CD) is a medically prescribed gluten free diet (GFD). Adoption of a strict GFD for life, is challenging and requires significant lifestyle change. Understanding the views and experiences of the coeliac community is imperative to improving the management and wellbeing of this patient group. These are results from a series of surveys of UK cohorts of the coeliac population, carried out by Coeliac UK between 2021 and 2022. There is a misunderstanding of the statement ‘gluten free’ among the UK coeliac community, with 61.5% unable to correctly identify the EU gluten free threshold of < 20 mg/kg and 40.6% believing gluten free to mean ‘zero gluten’ [1]. There is currently no standard legislation across Europe for the unintentional presence of allergens in food and precautionary allergen labelling (PAL), ‘may contain’ statements, in many cases remain voluntary. This has led to confusion and a distrust of such information by the coeliac community, so that only 48.0% of people with coeliac disease never include products with a ‘may contain gluten’ statement, 52.0% risking possible trace gluten ingestion [2]. ‘Not suitable for coeliacs’ was considered by 83.6% to be the most effective statement for explaining cross contamination risk from grains containing gluten [1]. Only 51.2% of people with CD reported that they hadn’t had any symptoms associated with their condition in the last 4 weeks [2]. Although 6 out of 10 people are either satisfied or very satisfied with the GFD, 73.7% consider it very or extremely important to have a therapeutic to replace the GFD and 86.1% to have an adjunct to the GFD, to protect them from risk of cross contamination or to help support the healing of their gut [3]. Although 61.7% indicated they would take part in a clinical trial for CD, 70.5% expressed their knowledge of what is involved in a clinical trial as being very poor to basic [3]. More than 70% of people with CD had prescribed medications in addition to the GFD and only 30% didn’t have another condition or complication [3]. A lifelong GFD is a significant undertaking. Although it is mostly self-managed there is reliance on many other stakeholders, policy makers, legislators, researchers, the food and pharma industries. PAL remains problematic and many people with CD continue to experience ongoing symptoms with the majority experiencing comorbidities or complications. There is interest in alternative therapeutics for CD, but we need to better inform the coeliac community about what is involved in clinical trials. These findings are limited to the coeliac population in the UK and should be explored further.
References
1. Coeliac UK’s Shopping Habits Survey 2022 (as yet unpublished)
2. Coeliac UK’s Dietary Preferences Survey 2021 (as yet unpublished)
3. Coeliac UK’s Clinical Trials and Therapeutics Survey 2021 (manuscript in writing)
Statements on current developments
concerning gluten analysis, clinical and legal
aspects
Update on regulatory issues of gluten
Hertha Deutsch
AOECS Codex Delegate, Austrian Coeliac Society, Vienna
Abstract
AOECS, the Association Of European Coeliac Societies, has Observer status in the Codex Alimentarius Commission and the Committees since 1992. In the past months, the most important work for coeliacs was done in the Codex Committee on Food Labelling (CCFL), it took place virtually from 27 September – 1 October 2021. The task was:
Revision of the General Standard for the Labelling of Pre-packaged Foods – Provisions relevant to Allergen Labelling
Already in July 2019, the Codex Alimentarius Commission requested the "Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens" to work on this issue. AOECS asked the Chair of this Expert group to confirm that "Cereals containing gluten…" will be kept as the first priority in the "list of hypersensitivities" in the Standard as it is since 1999 till today.
The task of the Expert group consists of part 1: "Review and validation of Codex priority allergen list through risk assessment" and part 2 "Review and establish threshold levels in foods of the priority allergens". In May 2021, the Expert group published their report regarding part 1 and concluded "…. the following should be listed as priority allergens: Cereals containing gluten (i.e., wheat and other Triticum species, rye and other Secale species, barley and other Hordeum species and their hybridized strains), ….". The existing text of the Standard is "Cereals containing gluten, i.e., wheat, rye, barley, oats, spelt or their hybridized strains and products of these".
At the CCFL session, new texts were proposed in the Standard: Definitions of allergen, food allergy, food intolerance and hypersensitivity. AOECS informed the Committee that coeliac disease is not an allergy, it is an immune-mediated disorder and proposed to correct the suggested texts accordingly: in the definition of "food allergy", the reference to coeliac disease should be deleted and in "hypersensitivity" non-IgE-mediated food allergy should be changed to non-IgE-mediated immune disorder e.g., coeliac disease. Because the general term "allergen" has already been established (precautionary allergen labelling, list of allergens etc.), AOECS suggested to extend the text "…. result either in an allergic reaction or in an immune-mediated disorder in certain individuals. "
The part 2 of the work of the Expert is not concluded, therefore this item will be discussed again at the next CCFL session in 2023.
Codex Committee on Nutrition and Foods for Special Dietary Uses (CCNFSDU)
At the session in 2019, CCNFSDU concluded that it premature to consider a proposal from AACCI addressed to CCMAS to delete "Gluten-free Foods" in the Codex Standard 234 - 199 and replace it with "Corn- and Rice-Based Gluten-Free Foods" and "Oat-Based Gluten-Free Foods". CCNFSDU agreed to wait for the completion of ring trial tests and to consider this matter at a future date when more information became available. This item has not been placed on the agenda after that session. The reports of the Codex Committees are published on the Codex website www.fao.org.
Perspectives and action plan of the PWG
Peter Koehler
Biotask AG, Esslingen, Germany
The Prolamin Working Group executive meeting and joint discussion held on 31 March 2022, led to the decisions and statements outlined below.
Action plan
I. Analytical
The PWG gliadin reference material is available from Arbeitsgemeinschaft Getreideforschung e.V. (Association of Cereal Research), Mr. Tobias Schumacher, Schuetzenberg 10, 32756 Detmold, Germany, E-mail: info@agf-detmold.de.
The price for one batch (100 mg) is 150 Euro.
Material for at least 4 years is still on stock.
AOAC doesn’t support an isolate as suitable reference material and suggests reference flour. Therefore, the future of PWG gliadin is unclear! Plans to prepare new PWG gliadin reference material must be reconsidered.
The collaborative study Determination of Gliadin as a Measure of Gluten in Food by R5 Sandwich ELISA RIDASCREEN® Gliadin Matrix Extension: Collaborative Study 2012.01 supervised by Katharina Scherf was successfully completed. The results have been published in J AOAC Int 2022; 105(2): 442–455. https://doi.org/10.1093/jaoacint/qsab148
II. Clinical
An international consortium of PWG-members and associated partners will set out to find answers within the scope of the ImmunoSafe-CeD project, funded by the EU Joint Programming Initiative - A healthy diet for a healthy life. Supervisor: Katharina Scherf.
The PWG keeps considering becoming a working group under the umbrella of the International Society For The Study Of Celiac Disease
III. Members, Policy
Michael Schumann from Charité (gastroenterology) in Germany has been suggested as a new member of the group and will be invited to the next meeting.
Sandra Denery, INRAE, Nantes, France, has been suggested to replace Olivier Tranquet but she declined due to other obligations.
Bob Anderson, Wesley Medical Research Ltd, Brisbane, Australia has been suggested as a new member, but he is poorly available and participation in person is difficult. An Open Access position paper or review of the PWG to a current topic is scheduled for 2023 or 2024.
Proceedings of this meeting will be available free of charge in electronic form from the PWG website (http://www.wgpat.com).
Next meeting: 2023
We are very pleased to announce the venue for our meeting:
Wageningen, The Netherlands
Hosts:
René Smulders, Twan America, Ingrid van der Meer, Peter Weegels
Wageningen University & Research
E-mail: rene.smulders@wur.nl
Time: 22nd – 23rd September 2023
Focus of the meeting:
Symposium: Organoids for coeliac disease diagnosis
Analytical aspects of gluten The meeting will be limited to 55 participants and attendance is by invitation only. Invitations will be sent by April 2023. Registration deadline will be June 15, 2023.
Very special thanks to the hosts for this kind invitation!
|
|
List of Participants
GROUP MEMBERS
Prof. Dr. Carlo Catassi
Università Politecnica delle Marche
Department of Pediatrics
Via Corridoni 11
60123 ANCONA, ITALY
Phone: +39 071 5962364
E-mail: c.catassi@staff.univpm.it
Prof. Dr. Fernando G. Chirdo
Universidad Nacional de La Plata
Facultad de Ciencias Exactas
Instituto de Estudios Immunologicos y
Fisiopatologicos - IIFP
Calle 47 y 115
1900 LA PLATA, ARGENTINA
Phone: +54 221 423 5 333 (Int 45)
E-mail: fchirdo@biol.unlp.edu.ar
Prof. Dr. Paul J. Ciclitira
University of East Anglia
Medical School
Bob Champion Building
James Watson Road
BR4 7UJ NORWICH
UNITED KINGDOM
Phone: +44 203 751 1104
E-mail: pciclitira@btinternet.com
Prof. Dr. Conleth Feighery
University of Dublin, Department of
Immunology, St. James’s Hospital
James’s Street
DUBLIN 8, IRELAND
Phone: +353 879969041
E-mail: cfighery@tcd.ie
Dr. Carmen Gianfrani
Institute of Biochemistry and
Cell Biology - CNR
Via Pietro Castellino 111
80131 NAPLES, ITALY
Phone: +39 081 6132224
E-mail: c.gianfrani@ibp.cnr.it
Prof. Dr. Peter Koehler
Biotask AG
Schelztorstraße 54-56
73728 ESSLINGEN, GERMANY
Phone: +49 711 31059068
E-mail: peter.koehler@biotask.de
Prof. Dr. Frits Koning
Leiden University Medical Centre, E3-Q
Department of Immunohaematology
and Bloodbank
Albinusdreef 2
2333 ZA LEIDEN, THE NETHERLANDS
Phone: +31 715 266673
E-mail: fkoning@lumc.nl
Prof. Dr. Knut Lundin
University of Oslo
Institute of Clinical Medicine
Postboks 1171, Blindern
0881 OSLO, NORWAY
Phone: +47 90980325
E-mail: knut.lundin@medisin.uio.no
Prof. Dr. Stefania Masci
University of Tuscia
Department of Agricultural and Forest
Sciences (DAFNE)
Via San Camillo de Lellis s.n.c.
01100 VITERBO, ITALY
E-mail: masci@unitus.it
Prof. Dr. Katharina Scherf
Karlsruhe Institute of Technology (KIT)
Institute of Applied Biosciences
Department of Bioactive and
Functional Food Chemistry
Adenauerring 20 a
76131 KARLSRUHE, GERMANY
Phone: +49 721 608 42929
E-mail: katharina.scherf@kit.edu
Prof. Dr. Dr. Detlef Schuppan
I. Medizinische Klinik und Poliklinik
Universitätsmedizin der Johannes
Gutenberg-Universität Mainz
Institut für Translationale Medizin
Langenbeckstraße 1
55131 MAINZ, GERMANY
Phone: +49 6131 177355/177356/177104
E-mail:
detlef.schuppan@unimedizin-mainz.de
Dr. René Smulders
Wageningen University & Research,
Plant Research
Droevendaalsesteeg 1
6708 PB WAGENINGEN,
THE NETHETRLANDS
Phone: +31 620298266
E-mail: rene.smulders@wur.nl
Dr. Olivier Tranquet
INRA
Rue de la Géraudière BP 71627
44316 NANTES, FRANCE
Phone: +33 2406 75027
E-mail: olivier.tranquet@inra.fr
Prof. Dr. Riccardo Troncone
University Federico II
Department of Pediatrics
Via Pansini 5
80131 NAPLES, ITALY
Phone: +39 3483132274
E-mail: troncone@unina.it
INVITED SPEAKERS
Dr. Twan America
Proteomics facility, BU Bioscience Wageningen University & Research, Wageningen Plant Research Droevendaalsesteeg 1
6708 PB
WAGENINGEN, THE NETHERLANDS
E-mail: twan.america@wur.nl
Mrs. Hertha Deutsch
Österreichische Arbeitsgemeinschaft
Zöliakie
Anton Baumgartner Straße 44/C5/2302
1230 VIENNA, AUSTRIA
E-mail: hertha.deutsch@chello.at
Dr. Heidi Urwin
Coeliac UK 3 rd Floor, Apollo Centre, Desborough Rd. HP112QW
HIGH WYCOMBE, BUCKS, UNITED KINGDOM
E-mail: Heidi.Urwin@coeliac.org.uk
GUESTS
Mr. Guglielmo Adinolfi
Starch Europe | Plant-Based Solutions 43 Avenue des Arts B-1040 BRUSSELS, BELGIUM
E-mail: guglielmo.adinolfi@starch.eu
Mr. Dave Almy
NEOGEN Corporation 620 Lesher Place 48912 LANSING, MI, USA
E-mail: dalmy@neogen.com
Dr. Guenther Augustin
Dr. Schär AG / SPA Winkelau 9 39014 BURGSTALL/POSTAL, ITALY
E-mail: guenther.augustin@drschaer.com
Dr. Emmanuelle Bancel
Université Clermont Auvergne-INRAE UMR1095 GDEC 63100 CLERMONT-FERRAND, FRANCE
E-mail: emmanuelle.bancel@inrae.fr
Dr. Francisco Barro Losada
Institute for Sustainable Agriculture Alameda del Obispo s/n 14004-CÓRDOBA, SPAIN
E-Mail: fbarro@ias.csic.es
Ms. Sofia Beisel
Deutsche Zöliakiegesellschaft e.V. Kupferstraße 36, 70565 STUTTGART, GERMANY
E-mail: sofia.beisel@dzg-online.de
Dr. Markus Brandt
Ernst Böcker GmbH & Co KG Ringstrasse 55-57 32427 MINDEN, GERMANY
E-mail: markus.brandt@sauerteig.de
Mr. Martin Candia
Romer Labs Division Holding GmbH Erber Campus 1 3131 GETZERSDORF, AUSTRIA
E-mail: martin.candia@dsm.com
Ms. Ángela Ruiz Carnicer
Universidad de Sevilla C/ Profesor García González 2 41012 SEVILLA, SPAIN
E-mail: acarnicer@us.es
Dr. Maria Cristina Comelli
Nemysis Limited Suite 4.01 Ormond Building 31-36 Ormond Quay Upper, Arran Quay D07 F6DC DUBLIN 7, IRELAND
E-mail: comelli@nemysisltd.com
Ms. Wieneke Dijk
INRAE – Unité BIA – Equipe Allergie Impasse Thérèse Bertrand-Fontaine 44000 NANTES, FRANCE
E-mail: wieneke.dijk@inrae.fr
Prof. Dr. Valentina Discepolo
University of Naples Federico II Department of Translational Medical Sciences Via Sergio Pansini 5 80131 NAPLES, ITALY
E-mail: valentina.discepolo@unina.it
Dr. Margareta Elding-Pontén
Fria Bröd AB Fältspatsgatan 12 421 30 VÄSTRA FRÖLUNDA, SWEDEN
E-mail: Margareta.Elding-Ponten@fria.se
Ms. Sophia Escobar-Correas
Agriculture and Food | CSIRO 306 Carmody Road ST. LUCIA QLD 4067, AUSTRALIA
E-mail: sophia.escobarcorreas@csiro.au
Ms. Blanca Esteban
Asociación de Celíacos y Sensibles Al Gluten, Comunidad de Madrid Calle Lanuza 19-bajo 28028 MADRID, SPAIN
E-mail: consultasdieta@celiacosmadrid.org
Mr. Richard Fielder
Bio-Check (UK) Spectrum House, Llys Edmund Prys St. Asaph Business Park LL170JA ST. ASAPH, UNITED KINGDOM
E-mail: richard@biocheck.uk.com
Ms. Maren Finke
Hermann Kröner GmbH Lengericher Straße 158 49479 IBBENBÜREN, GERMANY
E-mail: finke@kroener-staerke.de
Dr. Dana Gabrovská
Federation of the Food and Drink Industries of the Czech Republic (FFDI) Počernická 96/272 108 03 PRAHA 10, CZECH REPUBLIC
E-mail: gabrovska@foodnet.cz
Dr. Carlos Galera
Hygiena Diagnostica España Calle Cañada Real 31-35 41900 CAMAS, SEVILLA, SPAIN
E-mail: cgalera@hygiena.com
Ms. Alessandra Giuliano
Internal Medicine Unit University of Palermo 90127 PALERMO, ITALY
E-mail: alegiuliano94@gmail.com
Dr. Paul Jordan
Codexis, Inc. 200 Penobscot Drive CA 94063 REDWOOD CITY, USA
E-mail: paul.jordan@codexis.com
Mr. Max Kendall
Nemysis Limited Suite 4.01 Ormond Building 31-36 Ormond Quay Upper, Arran Quay D07 F6DC Dublin 7, IRELAND
E-mail: kendall@nemysisltd.com
Dr. Katharina Kessler
Nemysis Limited Suite 4.01 Ormond Building 31-36 Ormond Quay Upper, Arran Quay D07 F6DC Dublin 7, IRELAND
E-mail: kessler@nemysisltd.com
Mrs. Tunde Koltai
Hungarian Coeliac Society Palanta utca 11 1025 BUDAPEST, HUNGARY
E-mail: tunde.koltai@gmail.com; coeliac@t-online.hu
Dr. Götz Kröner
Hermann Kröner GmbH Lengericher Str. 158 49479 IBBENBÜREN, GERMANY
E-mail: kroener@kroener-staerke.de
Ms. Mélanie Lavoignat
Université Clermont Auvergne-INRAE UMR1095 GDEC 63100 CLERMONT-FERRAND, FRANCE
E-mail: melanie.lavoignat@inrae.fr
Dr. Barbara Lexhaller
Karlsruhe Institute of Technology (KIT) Institute of Applied Biosciences Department of Bioactive and Functional Food Chemistry Adenauerring 20 a 76131 KARLSRUHE, GERMANY
E-mail: barbara.lexhaller@kit.edu
Mr. Martin Lok
División Química de los Alimentos CIATI, ARGENTINA
E-Mail: martinl@ciati.com.ar
Dr. Gabriela Lopez Velasco
3M Food Safety 3M Center 0260-B-01 55144 ST. PAUL, MN, USA
E-mail: glopez3@mmm.com
Dr. Patrick Mach
3M Food safety 3M Center, 0260-B-01 55144 ST. PAUL, MN, USA
E-Mail: pamach1@mmm.com
Prof. Dr. Pasquale Mansueto
Internal Medicine Unit University of Palermo 90127 PALERMO, ITALY
E-mail: pasquale.mansueto@unipa.it
Dr. Kathleen Molnar
Codexis, Inc. 200 Penobscot Drive CA 94063 REDWOOD CITY, USA
E-mail: kathleen.molnar@codexis.com
Mr. Steffen Muench
Evergrain, LLC 1209 Orange Street DE 19801 WILMINGTON, USA
E-mail: Steffen.Muench@Everingredients.com
Dr. Susanna Neuhold
Associazione Italiana Celiachia Via Caffaro, 10 16124 GENOVA, ITALY
E-mail: alimenti@celiachia.it
Ms. Marie-Christin
Norwig Leibniz-Institute for Food Systems Biology at the Technical University of Munich (Leibniz-LSB@TUM) Lise-Meitner-Str. 34 85354 FREISING, GERMANY
E-mail: m.norwig.leibniz-lsb@tum.de
Dr. Luisa Novellino
Associazione Italiana Celiachia Via Caffaro, 10 16124 GENOVA, ITALY E-mail: lnovellino@celiachia.it
Dr. Adrian Rogers
Bio-Check (UK) Spectrum House, Llys Edmund Prys St. Asaph Business Park LL170JA ST. ASAPH, UNITED KINGDOM
E-mail: adrian@biocheck.uk.com
Ms. Cristina Romero
Eurofins Ingenasa S.A. c/Hermanos García Noblejas, 39 28037 MADRID, SPAIN
E-mail: cromero@ingenasa.com
Ing. Jana Rysová
Food Research Institute Prague Radiová 1285/7 102 00 PRAHA 10, CZECH REPUBLIC
E-Mail: j.rysova@vupp.cz
Mr. Stefan Schmidt
R-Biopharm AG An der neuen Bergstraße 17 64297 DARMSTADT, GERMANY
E-mail: st.schmidt@r-biopharm.de
Mr. Zachary Schwingel
NEOGEN Corporation 620 Lesher Place 48912 LANSING, MI, USA
E-Mail: Zschwingel@neogen.com
Ms. Verónica Segura Montero
Universidad de Sevilla C/ Profesor García González 2 41012 SEVILLA, SPAIN
E-Mail: vsegura@us.es
Dr. Juan Ignacio Serrano-Vela
Asociación de Celíacos y Sensibles Al Gluten, Comunidad de Madrid Calle Lanuza 19-bajo 28028 MADRID, SPAIN
E-mail: nachoserrano@celiacosmadrid.org
Prof. Dr. Edurne Simón
University of the Basque Country Paseo de la Universidad, 7 1006 VITORIA-GASTEIZ, SPAIN
E-mail: edurne.simon@ehu.es
Ms. Charlene Taylor
NEOGEN Europe Ltd. The Dairy School, Auchincruive KA6 5HU AYR, SCOTLAND UNITED KINGDOM
E-mail: ctaylor@neogen.com
Dr. Karoline Terberger
Böcker Sauerteig GmbH & Co. KG Ringstraße 55-57 32427 MINDEN, GERMANY
E-mail: karoline.terberger@sauerteig.de
Dr. Catherine Torgler
Hygiena Diagnostica España Calle Cañada Real 31-35 41900 CAMAS, SEVILLA, SPAIN
E-mail: ctorgler@hygiena.com
Ms. Claire Van der Aa
Evergrain, LLC 1209 Orange Street 19801 WILMINGTON, DE, USA
E-mail: Claire.Vanderaa@everingredients.com
Dr. Patrizia Vaccino
Council for Agricultural Research and Economics (CREA) S.S. 11 per Torino, Km 2,5 13100, Vercelli, ITALY
E-mail: patrizia.vaccino@crea.gov.it
Dr. Olimpia Vincentini
Department of Food Safety, Nutrition, and Veterinary Public Health Unit of Human Nutrition and Health Viale Regina Elena 299-00161 ROME, ITALY
E-mail: olimpia.vincentini@iss.it
Dr. Peter Weegels
Laboratory of Food Chemistry Wageningen University & Research Bornse Weilanden 9 6708 WG WAGENINGEN, THE NETHERLANDS
E-mail: peter.weegels@wur.nl
Dr. Paul Wehling
ChemStats Consulting 3230 Taylor Street Ne 55418 MINNEAPOLIS, MN, USA E-mail: paul@chemstats.com Dr. Thomas Weiss R-Biopharm AG An der neuen Bergstraße 17 64297 DARMSTADT, GERMANY
E-mail: t.weiss@r-biopharm.de
Dr. Tricia Windgassen
Codexis, Inc. 200 Penobscot Drive 94063 REDWOOD CITY, CA, USA E-mail: tricia.windgassen@codexis.com
Ms. Majlinda Xhaferaj Karlsruhe
Institute of Technology (KIT) Institute of Applied Biosciences Department of Bioactive and Functional Food Chemistry Adenauerring 20 a 76131 KARLSRUHE, GERMANY
E-mail: majlinda.xhaferaj@kit.edu
Ms. Qianying Xu
University of Manchester Division of Infection, Immunity and Respiratory Medicine Oxford Rd. MANCHESTER M13 9PL, UNITED KINGDOM,
E-mail: qianying.xu-2@postgrad.manchester.ac.uk
Impressum
Proceedings of the 35th Meeting
WORKING GROUP
on PROLAMIN ANALYSIS and TOXICITY
31 March – 01 April 2022
Online
This work including all parts is subject to copyright. All rights are reserved and any
utilisation is only permitted under the provisions of the German Copyright Law.
Permissions for use must always be obtained from the publisher. This is in particular
valid for reproduction, translation, conversion to microfilm and for storage or
processing in electronic systems.
Scientific Organisation and Host
Prof. Dr. Peter Koehler
Lecturer for Food Chemistry at Technical University of Munich Employee of biotask AG Schelztorstraße 54-56, 73728 ESSLINGEN, GERMANY
Phone: +49 711 31059068;
Fax: +49 711 31059070
E-mail: peter.koehler@tum.de
Cover picture
Cover design* Peter Koehler
The picture shows part of the MALDI-TOF mass spectra of PWG-gliadin
© Peter Koehler 2022
|


