Proceedings of the 25th Meeting
Working Group on Prolamin Analysis
and Toxicity (PWG)
Edited by Peter Koehler
German Research Centre for Food Chemistry, Freising
Verlag Deutsche Forschungsanstalt für Lebensmittelchemie - 2012
Preface
The 25th meeting of the Working Group on Prolamin Analysis and Toxicity (PWG) was held at Classic Congress Hotel, Fellbach, Germany, from September 29 to October 2, 2011. This time the PWG was hosted by the German Coeliac Society, namely Sofia Beisel, Judith Suck and Andreas Abbrecht, who were present during the entire meeting. Martin Stern, chairman of the PWG, welcomed the group, the invited speakers, and the participants from industry (cereal starch producers, producers of gluten-free food, manufacturers of kits for gluten quantification), members of research institutes as well as delegates from international coeliac societies.
At the 24th meeting 2010 in Ancona the executive members agreed to establish food technology, in particular gluten technology, as an additional focus of the group and this was reflected in the programme, which included a symposium on gluten in food technology. Two recognised experts in this field shared results of their research with the audience. In addition, new findings on proteins triggering coeliac disease and, possibly, related intolerances were reported in the clinical session for the first time. This might provide new insights into the pathway of innate immunity as well as a better understanding of gluten sensitivity.
As the new chairman of the group I am grateful to all participants for their active contributions, in particular to Sofia Beisel and Judith Suck from the German Coeliac Society for the excellent organisation of the meeting. The Swabian feeling with wine and excellent food typical for the south-west of Germany was an important point that contributed to an exciting meeting. I express my gratitude to all friends, colleagues, sponsors and participants for their inspiration and support.
Freising, March, 2012 Peter Koehler
I. Executive Summary
The meeting focused on food technological issues of gluten, in particular on the production of gluten-free baked goods.
Analytical reports
Seven reports were given on gluten antibody specificity, quantification of gluten by ELISA, the analysis of oats and buckwheat, the gluten load in gluten-free diets and peptidases for gluten degradation. In particular the standardisation of the R5 ELISA with different organisations such as American Association of Cereal Chemists International (AACCI) and Association of Official Analytical Chemists (AOAC) is one goal to be achieved in the year 2012. In addition, problems in analysis of deamidated gluten were addressed.
Clinical reports
The seven reports addressed issues in the diagnosis of coeliac disease by antibody tests, risk assessment of gluten exposure, refractory coeliac disease and the role of the innate immune system in the pathogenesis. New findings on amylase-trypsin-inhibitors (ATI) provided information on innate immunity and could, possibly, be an explanation for the phenomenon of gluten sensitivity, which has been poorly understood up to date.
Symposium: gluten in food technology
Two presentations of experts in this field were given, showing the possibilities and the limitations of food technology in the production of gluten-free foods, in particular bread. Considerable progress has been made in the last years and a number of novel gluten-free foods has entered the market. However, some gluten-free products are still of poor textural and sensory quality compared to their gluten-containing counterparts.
IV. Analytical research reports
Differential immunoreactivity of selected monoclonal
antibodies towards a reference gliadin
Renate van Eckert1, Judy Bond1, Paul J. Ciclitira2, H. Julia Ellis2, Pisana Rawson1,
Christoph Klein3, Martin Stern4, T. William Jordan1
1 Centre for Biodiscovery and School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand
2 King’s College London, Division of Diabetes and Nutritional Sciences, Rayne Institute, St. Thomas’ Hospital, London, England
3 European Commission, Directorate-General, Joint Research Centre (JRC), Institute for Health and Consumer Protection (IHCP), Via E. Fermi, 21027 Ispra, Italy
4 University Children’s Hospital, Tübingen, Germany
Abstract
The reactivity of three selected antibodies was investigated with a gliadin material (PWG-gliadin) after two-dimensional electrophoresis (2-DE) and transfer of the proteins with Western Blot onto PVDF-membranes. Fluorescence labelling was used to differentiate the reacting and non-reacting proteins. The gliadin material was fluorescence labelled with Cy3 and then separated by 2-DE. After Western Blot to PVDF-membranes the proteins were incubated with anti-gliadin mouse antibodies 401.21, PN3 and R5 respectively. The reacting proteins were detected with a Cy5 fluorescence labelled anti-mouse antibody. Differential scanning at specific wavelengths for Cy3 and Cy5, respectively, yielded the 2-DE pattern of the reacting and non-reacting proteins in the same membrane. Antibodies 401.21, PN3 and R5 each detected different protein sets of the gliadin material. Thus these antibodies can yield different measurements of gluten quantity, when used in ELISA for the determination of gluten. The findings help to explain why the results for the gluten content of the same test specimen were dependent on the ELISA test kits used and how standardisation can contribute to establishing reliable gluten measurements.
Introduction
Coeliac patients need to adhere to a strict gluten-free diet for their entire life in order to avoid symptoms. For this reason a reliable detection method for gluten is needed. The mainstays in gluten analysis are ELISA methods because of their sensitivity and specificity. We had shown, however, that different gliadin preparations produced different responses in gluten ELISA systems [1], which were based on the antibody developed by Skerritt and Hill [2], and that the determination of gluten with different ELISA test kits yielded sometimes very different gluten quantities [3]. We assumed that the various antibodies used in ELISA test kits detected different individual proteins, but we were not able to prove this hypothesis. Our comparison of the reaction of three selected monoclonal antibodies with a gliadin material separated by 2-DE confirmed this assumption.
Material and methods
The reaction of the following primary antibodies was investigated:
1. Monoclonal antibody (mAb) 401.21: IgG1 mouse mAb, developed against gliadin by Skerritt and Hill [2], kindly provided by the company Vital Diagnostics Pty Ltd, Australia.
2. PN3-mAb: IgG1 mouse mAb, developed against a 19-mer peptide of A-gliadin by Ellis et al. [4], kindly provided by the research group of Prof. Dr. Paul Ciclitira, U.K..
3. R5-mAb: IgG2b mouse mAb, developed against secalin [5], kindly provided by Operon S.A., Cuarte de Huerva, Spain, via the late Dr. Enrique Méndez.
The gliadin material used was “PWG-gliadin” (short for Prolamin Working Groupgliadin). It had been extracted with 60 % (v/v) ethanol from 28 commonly used European bread wheat varieties [6].
We used Fluorescent labelling dye: CyDyeTM DIGE Fluor CyTM3 (Cy3), minimal dye (GE-Healthcare, 25-8010-83 ) for the Cy3-fluorescence labelling of PWG-gliadin.
We used ECL Plex goat anti-mouse IgG, labelled with fluorescent dye CyDyeTM DIGE Fluor CyTM5 (Cy5) (GE-Healthcare, PA 45009) as a secondary antibody for all three primary mouse mAbs.
Details of the labelling procedure of PWG-gliadin, the electrophoresis, Western Blot, antibody reaction and fluorescence scanning can be found in van Eckert et al. [7]. We used a very stringent washing regime and a high concentration of bovine serum albumin in the blocking buffer to avoid unspecific reactions. We allocated the reacting proteins to gliadin and glutenin sub-groups on the basis of their apparent molecular weight (known from our own results and from published data).
With the fluorescence technique used we were able to detect the reacting and nonreacting proteins in the same membrane and to monitor the protein pattern at any stage of the electrophoresis, blotting and antibody procedures without interference or additional stain or the need of a control run of a second gel.
Results
The three different antibodies showed a different reaction with different sets of individual proteins of the gliadin material.
MAb 401.21 reacted mainly with proteins of an approximate molecular weight of 60,000 and above. It showed a reaction with HMW-glutenin subunits, presumably with LMW-glutenins, with ω-gliadins and – to a small degree – with α- and γ-gliadins. The reaction of proteins in the HMW-area contributed most to the entire antibody reaction.
PN3-mAb reacted mainly with proteins of an apparent molecular weight of 30,000 and higher, which corresponds to the apparent molecular weight of α-gliadins.
R5-mAb reacted strongly with α- and γ-gliadins, especially those with a low isoelectric point. The reaction with γ-gliadins seemed to be the strongest. R5-mAb also reacted with proteins of an apparent molecular weight of about 50,000 and 75,000 and higher (probably ω-gliadins).
The 2-DE protein pattern of PWG gliadin was the same through all procedures applied. The spots seemed slightly enlarged after the blot of the proteins from gel to membrane. Some proteins in the migration area of ω-gliadins and LMW-glutenins were less intense after the completed antibody reaction. The results are documented in detail in van Eckert et al. [7].
Discussion
The fluorescence technique used was an effective method for the comparison of the reactivity of the three selected antibodies. It was more sensitive than the commonly used Coomassie Blue Stain and had the advantage that there was no additional stain necessary in order to measure the protein and/or antibody spots. All three antibodies being monoclonal mouse antibodies, we were able to detect them with the same secondary Cy5 labelled anti-mouse antibody. Thus it was possible to minimise influences from staining and to avoid gel-to-gel variation, as the Cy3 labelled gliadin and the Cy5 labelled reacting proteins were detected by differential scanning at the same time on the same membrane.
The fact that some proteins in the ω-gliadin and LMW-glutenin area were less intense after the completed antibody reaction agrees with findings from other authors: Hurkman and Tanaka [8] observed a reduction in colloidal Coomassie Blue G-250 stained proteins, when they were kept in water for 3 - 24 hours. Van den Broeck et al. [9] reported a reduction of ω-gliadins, LMW-glutenins and some α-gliadins, when Coomassie stained gels were destained in 10 % ethanol/7.5 % acetic acid.
Each of the three antibodies detected different sub-types of gluten proteins to a different degree. These findings demonstrate that the amount of gluten detected is dependent on the antibody and on the reference material used. MAb 401.21 shows a strong reaction with HMW-glutenins. This result explains why gliadin preparations extracted by H. Wieser showed a relatively low reaction in gluten assays based on mAb 401.21 [1,6]. The gliadin preparations produced by H. Wieser were obviously very pure in regard to their gliadin content and did not contain many HMW-glutenins. Now we can also explain why RM 8418, a gluten preparation made from a Canadian spring wheat, reacted to a higher degree than PWG-gliadin in assays based on mAb 401.21 [6]. RM 8418 is composed of gliadins and glutenins, and the glutenins contribute strongly to the assay response. PWG-gliadin, however, has been extracted with 60 % ethanol from wheat flour, and the gliadins are strongly enriched.
PN3-mAb seems to recognise distinctively α-gliadins. This fits well together with the fact that PN3-mAb was raised against a peptide from A-gliadin, an α-gliadin. It was suggested that this mAb reacted mainly with the epitope QQQPFP [4], which is found in α-, but not in γ-gliadins.
R5-mAb recognises the epitope with the sequence QQPFP the greatest [10]. It also reacts with homologous repeats like QQQFP, LQPFP and QLPFP [11]. The QQPFPepitope occurs repeatedly in α-, γ- and ω-gliadins. It has only one amino acid less than the main reactant QQQPFP of mAb PN3, and it occurs more often in γ- and ω-gliadins [12]. This is in agreement with our results, where mAb R5 showed a high reaction with γ-gliadins. The diffusion of ω-gliadins from the membrane during the incubation and washing steps of the antibody reaction might have diminished their response.
In summary each antibody investigated detected a different set of gliadin or glutenin proteins. This difference in reactivity with different individual proteins explains why different gluten amounts were obtained in the past, when different ELISA test systems were employed or when different reference materials were used. The results emphasise the importance of a well-characterised reference material, and of an antibody which detects the proteins that are to be determined representatively. As it has been found that glutenins carry coeliac toxicity as well [13], it is desirable that antibodies are available which detect glutenins as well as gliadins. The detection of gluten with gliadin and glutenin antibodies, which react to a similar degree, in combination with a well-characterised reference material might be the approach of the future.
Acknowledgements
We wish to thank Dr. Herbert Wieser for providing gliadin preparations and for useful discussions, Prof. Dr. Paul Ciclitira and Dr. Julia Ellis for providing mAb PN3, Vital Diagnostics Pty Ltd for providing mAb 401.21, Operon SA for providing mAb R5 and Dr. Heinz Schimmel (Institute for Reference Materials and Measurements of the European Commission Joint Research Centre, Geel, Belgium) for funding (Extended characterisation study of Gliadin from European wheat, B030333).
References
1. Van Eckert R. Methodological and practical experience in gluten analysis. Proceedings of the 7th meeting of the working group on prolamin analysis and toxicity, November 4-6, 1992; Schloß Weitenburg, Germany, pp. 83-86.
2. Skerritt JH, Hill AS. Monoclonal antibody sandwich enzyme immunoassays for determination of gluten in foods. J Agric Food Chem 1990; 38: 1771-1778.
3. Van Eckert R, Scharf M, Wald T, et al. Determination of proteins with ELISAMethods: Doubtful quantitative results? In: Amado R., Battaglia R. (Eds.)
Proceedings of EURO FOOD CHEM IX, 1997, Interlaken, Switzerland. FECS Event No. 220 (Vol.1): 263-268.
4. Ellis HJ, Rosen-Bronson S, O’Reilly N, et al. Measurement of gluten using a monoclonal antibody against a coeliac toxic peptide of A-gliadin. Gut 1998; 43: 190-195.
5. Sorell L, López JA, Valdés I, et al. An innovative sandwich ELISA system based on an antibody cocktail for gluten analysis. FEBS Letters 1988; 439: 46-50.
6. Van Eckert R, Berghofer E, Ciclitira P J, et al. Towards a New Gliadin Reference Material - Isolation and Characterisation. J Cereal Sci 2006; 43: 331- 341.
7. Van Eckert R, Bond J, Rawson P, et al. Reactivity of gluten detecting antibodies to a gliadin reference material. J Cereal Sci 2010; 51: 198-204.
8. Hurkman WJ, Tanaka CK. Improved methods for separation of wheat endosperm proteins and analysis by two-dimensional gel electrophoresis. J Cereal Sci 2004; 40: 295-299.
9. Van den Broeck HC, America AHP, Smulders MJM, et al. Staining efficiency of specific proteins depends on the staining method: Wheat gluten proteins. Proteomics 2008; 8: 1880-1884.
10. Valdés I, Garcia E, Llorente M, et al. Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur J Gastroenterol Hepatol 2003; 15: 465-474.
11. Kahlenberg F, Sanchez D, Lachmann I, et al. Monoclonal antibody R5 for detection of putatively coeliac-toxic gliadin peptides. Eur Food Res Technol 2006; 222: 78-82.
12. Osman AA, Uhlig HH, Valdés I, et al. A monoclonal antibody that recognizes a potential coeliac-toxic repetitive pentapeptide epitope in gliadins. Eur J Gastroenterol Hepatol 2001; 13: 1189-1193.
13. Dewar DH, Amato M, Ellis HJ, et al. The toxicity of high molecular weight glutenin subunits of wheat to patients with coeliac disease. Eur J Gastroenterol Hepatol 2006; 18: 483-491.
Collaborative study on gluten determination using
sandwich and competitive R5 ELISA kits
Peter Koehler1, Theresa Schwalb1, Clyde Don2
1 German Research Centre for Food Chemistry, Freising, Germany
2 Foodphysica, Driel, The Netherlands
Introduction
In September 2009 it was agreed with the Protein Technical Committee of the AACC International to organise a collaborative study to check the performance of the sandwich ELISA RIDASCREEN® Gliadin R7001 for raw and processed food materials as well as of the RIDASCREEN® Gliadin competitive R7021 for the determination of partially hydrolysed prolamin in fermented food. Prolamin is the alcohol-soluble portion of gluten. A definition of gluten and prolamin with respect to coeliac disease is given in the “Codex Standard for foods for special dietary use for persons intolerant to gluten (Codex Stan 118 – 1979)” [1] from 2008 and for gluten in the “Commission Regulation (EC) No 41/2009” [2]. The collaborative study was coordinated by Prof. Dr. Peter Koehler in close collaboration with AACC International (Dr. Clyde Don, chairman of the Protein Technical Committee). R-Biopharm provided sandwich and competitive ELISA kits for the study. In a first stage a mini-collab was performed in order to see whether the collaborative study design would perform as expected. The aims were to validate the sandwich as well as the competitive ELISA for prolamin/gluten quantitation, using the inter-lab guidelines for AACC International Approved Methods. As there is a close resemblance in the general set-up of a collaborative study, approval by ICC and AOAC can follow. This is a preliminary report on the practical part of the study. Statistical evaluation of the data has not been carried out yet.
Laboratories
Participating labs were selected from all over the world. The labs were required to be familiar with immunological tests, if possible, with the R5 ELISA. A separate room for the analysis of gluten-free foods was required and staff and time had to be provided for the study. Looking at the requirements of laboratories, sample set and low concentration of the analyte (mg/kg level) it was advised by the Protein & Enzymes Committee of AACC International to start with a mini-collaborative study. The minicollaborative study was done during the first part of 2011. After discussion of the results it was decided that the set-up just needed minor modifications, and the planning of the full collaborative study went on as scheduled. The time period was six weeks, and the study was carried out from August 1 to September 15, 2011. 16 labs designated A to P were selected from Argentina, Austria, Belgium, Canada, Finland, Germany (2), Hungary, Ireland, Italy, New Zealand, Sweden, Switzerland, and USA (3).
Samples and sample preparation
Two sample series were prepared. Series 1 contained non-hydrolysed gluten and was analysed with the Sandwich ELISA, whereas in samples of series 2 partially hydrolysed gluten was present, which had to be analysed by competitive ELISA. Samples are compiled in Tab. 1.
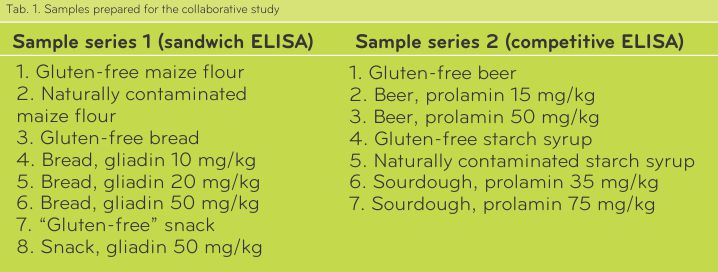
Samples of series 1 were differently heat-treated. Maize flour was not heat-treated, bread was moderately heat-treated, and the extruded snack was heavily heat-treated. Bread and snack were based on gluten-free maize flour, to which wheat flour with a defined gliadin content (determined by HPLC) was added. The analyses showed that the “Gluten-free” snack contained gluten contamination, probably coming from the production line. Samples of series 2 were differently prepared. Gluten-free beer made from sorghum was used as a base material, which was spiked to a defined prolamin concentration with a peptic-tryptic hordein digest. Gluten-free maize starch syrup and contaminated wheat starch syrup were obtained by suppliers. Contaminated sourdough was prepared by mixing dried, gluten-free quinoa sourdough and rye sourdough with a defined gluten content (determined by competitive R5 ELISA).
Sample presentation to labs
Initial considerations were that two independent replicates for each sample should be done. This was achieved by dividing the samples into two parts and presenting each part as an independent, differently coded sample to the labs. Thus, duplicates were present as regular samples enabling completely independent duplicate determinations for each sample. Therefore, 16 samples had to be analysed by the sandwich and 14 samples by the competitive ELISA. Different coding of samples was used for each lab.
Assay protocol
Assay protocols for both the sandwich and competitive ELISA were provided and labs had to follow the instructions carefully. In particular, it was described, in which cases samples had to be diluted and how dilutions had to be carried out. All optical densities had to be recorded, as well as prolamin and gluten (= prolamin x 2) concentrations calculated by R-Biopharm’s software “RIDA®SOFT”. Finally, the labs provided all results in a report template.
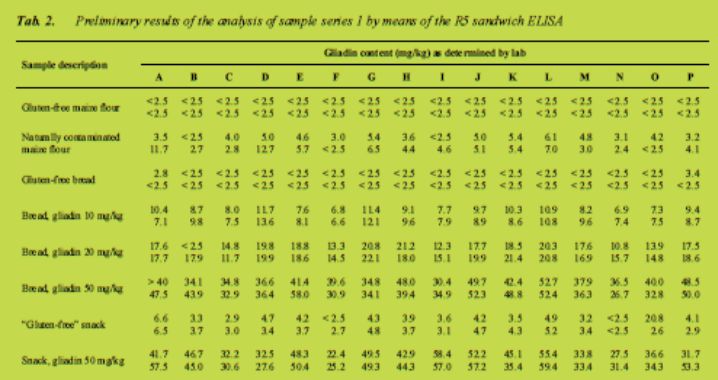
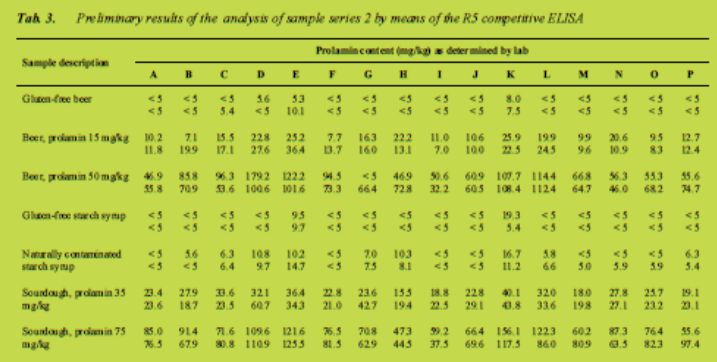
Preliminary results
The results as they were provided by the participants are compiled in Tab. 2 (sandwich ELISA) and Tab. 3 (competitive ELISA). They have to be regarded as preliminary as no statistical treatment of the data, such as outlier test, interpolation of values outside of the calibration curve or development of a calculation model, has been carried out so far. In the sandwich assay mean coefficients of variation were ± 18 % for samples above 10 mg prolamin/kg and ± 26 % for samples below 10 mg/kg. The competitive assay performed somewhat worse with a mean coefficient of variation of 30 %. To give an idea of the scope of possible precision (RSDr and RSDR) we refer to AOAC 999.19 [3]. So far it seems that the results of the current method used in this 2011 collaborative study remained within the range of results found previously, perhaps they were even better for the important 20 mg/kg Codex threshold value. The more detailed statistical treatment of the dataset is currently underway.
Conclusion
The collaborative study has shown that wheat flour with defined gliadin content can be used to produce gluten-containing incurred material. Heating of gliadin does not affect its reactivity with the R5 antibody. Furthermore, peptic-tryptic hordein hydrolysate is suited to produce spiked samples from fermented foods. Regarding the performance of the ELISA kits, the sandwich assay appears to provide higher gliadin contents after dilution as compared to direct measurement of extracts. Both the sandwich and the competitive R5 ELISA are suitable methods to determine the prolamin content of different foods and are able to quantify low amounts of gluten.
References
ALINORM 08/31/26, Appendix III (2008) Draft revised codex standard for foods for special dietary use for persons intolerant to gluten. Joint FAO/WHO Food Standards Programme. Codex Alimentarius Commission WHO: Rome.
Commission Regulation (EC) No 41/2009 of 20 January 2009 concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten.
Official Methods of Analysis of AOAC INTERNATIONAL, method 991.19.
Food-related strategies towards reduction of gluten
intolerance and gluten sensitivity
Luud J.W.J. Gilissen1, Hetty C. van den Broeck1, Diana M Londono1, Elma M.J.
Salentijn1, Frits Koning2, Ingrid M. van der Meer1, Marinus J.M. Smulders1
1 Plant Research International (PRI), Wageningen University and Research Centre, Postal Box 16, 6700 AA Wageningen, The Netherlands
2 Department of Immunohematology and Blood Transfusion, Leiden University Medical Centre (LUMC), Leiden, The Netherlands
Around 1 % of the Western population suffers from coeliac disease (CD), a foodrelated inflammatory disorder of the small intestine caused by the ingestion of gluten in genetically predisposed individuals. This prevalence is still increasing [1,2]. Recently, a new and less well defined gluten (or wheat) related syndrome has emerged that seems to be unrelated to coeliac disease, named gluten sensitivity (GS). The socalled ‘gluten-free diet’ appears to improve significantly the health condition of these GS patients. In some studies, a direct correlation was found with gluten consumption [3], whereas other authors pinpoint on protein compounds that are co-extracted with gluten, such as amylase trypsin inhibitors (ATIs) [4]. The prevalence of GS is estimated at 5 - 10 % of the western population [5], but a clear definition is lacking, and no biomarkers and epidemiological data are available as well to confirm this percentage anyhow. However, the fact that the gluten-free market goes mainstream and is growing to several billion Euro sales annually reflects a steady trend that goes beyond coeliac disease [6].
The major difference between CD and GS is in the small intestine where cases of GS do not show the CD-specific villous atrophy. Other symptoms of CD and GS are similar and are highly diverse in both, including chronic abdominal pain, diarrhoea, and growth retardation in children, and chronic fatigue and headache, bowel complaints, reduced fertility, dermatitis herpetiformis, osteoporosis, nerve and brain (behaviour) disorders, increased risk of intestinal cancer in adults, to mention the most common ones. This wide variety of symptoms largely hampers good diagnosis. As a result, only 10 - 20 % of the CD population has been properly diagnosed, as will also be an unknown but possibly minor fraction of the GS population. This implies that the vast majority of the individuals with CD and GS are unaware of their disease. They continue their daily consumption of large amounts of gluten and worsen their health status and health perspectives, which is a major concern. The high food industrial qualities of wheat gluten have led, in recent decades, to a steady increase in its food-industrial applications. A survey in Australia of more than 10,000 supermarket items detected wheat in almost 30 % of labelled products [7]. In some of these products, the connection to wheat was visible and even proactivelymarketed; in other products, it was invisible. The latter group consisted not only of processed foods, but also foods that are not commonly associated with wheat, such as canned vegetables, milk, meat and even seafood and medicines; obviously, a big problem for individuals with CD and GS. Therefore, because of the apparent increase in the prevalence of wheat- and gluten-related symptoms, new applications of wheat gluten (in natural or modified forms), particularly in non-cereal-based food products should be considered deliberately, and the current use of wheat and gluten in saleable foods should be re-evaluated. Labelling of packed food products (according to Directive 2003/89/EC) [8] is helpful, but only for diagnosed individuals. As mentioned, these form only a minority of the patients. This creates a challenge. The question now arises how the food industrial quality of wheat and its gluten can be maintained while reducing or, even better, eliminating negative health effects.
Two strategies can be put forward:
1. Reduction of (coeliac) immunogenic proteins in regular wheat- and glutencontaining foods. As the induction of CD appears to be related to, amongst others, the dose of exposure to gluten-derived epitopes, we assume that every reduction in the consumption of harmful (CD-immunogenic) gluten will contribute to a general reduction of the prevalence of the disease(s) and of symptom severity in the population. This will, therefore, in time, benefit the general population, including the non- and wrong-diagnosed groups of CD and GS individuals.
2. Production of guaranteed safe and healthy foods for individuals that are already diagnosed with CD and have to follow a life-long gluten-free diet. Such food products will also be of benefit for people with GS.
Strategy 1 can be performed in two ways:
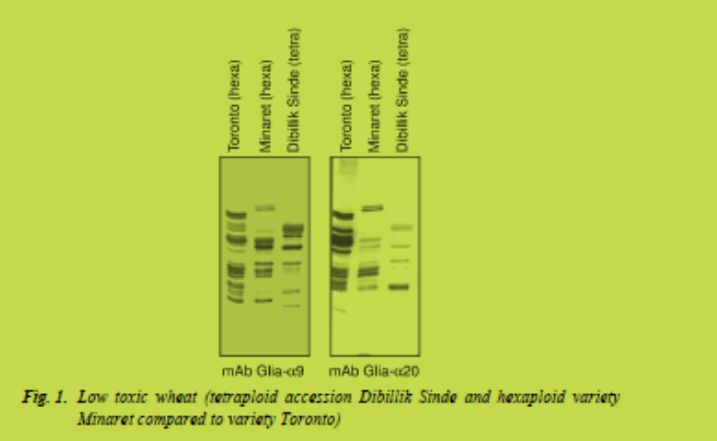
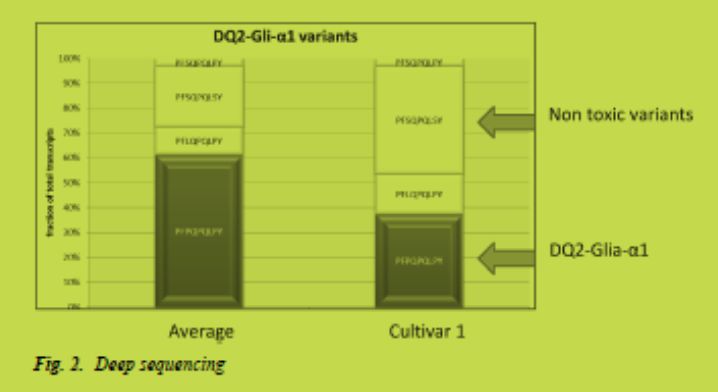
a) The systematic application of well-characterised low CD-immunogenic wheat varieties, which are currently under development [9,10] (Fig. 1). To achieve this goal, low CD-immunogenicity with regard to coeliac disease epitopes should become an additional wheat breeders’ aim. The currently developed immunological and molecular (e.g. deep 454-sequencing of expressed gluten genes, Fig. 2) tools for quantification of toxicity and for molecular marker-assisted breeding (Salentijn et al., in preparation) will be very helpful in the development of low CD-immunogenic wheat varieties.
Until being mainstream, such varieties will need to be processed in separate and strictly controlled production lines. This is a long-term approach.
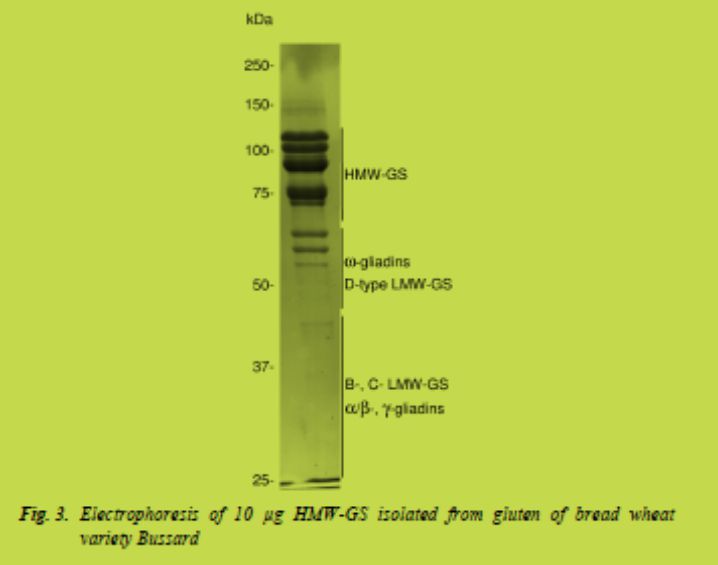
b) The general reduction of gluten in food products, comparable to the current goals of reduction of salt, fat and carbohydrates. This may include the development of technologically more efficient but less toxic gluten. With regard to industrial and technological quality characteristics, the glutenin component of gluten is much more relevant than the gliadin component. As the gliadins contain most of the coeliac disease epitopes, separation of specifically the glutenin fraction from the gluten may result in an economically and technologically profitable product with significantly reduced CD immunogenicity (van den Broeck et al. in preparation) (Fig. 3). This approach requires a change in the current industrial gluten production.
Strategy 2 may include:
a) The application of alternative processing techniques that eliminate (break down) the CD epitopes, such as sourdough fermentation [11,12].
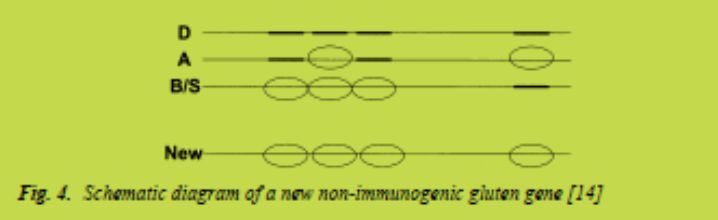
b) The production of completely safe gluten proteins, either recombinant or by processing, based on currently gained knowledge on the elimination of the toxic fragments (Fig. 4) [13,14].
c) Alternative cereals which are safe and also may provide sufficient technological properties. Among these cereals, oats are currently the best possible replacement for wheat, rye and barley. According to EC Regulation 41/2009 [15], oat products containing less than 20 ppm gluten are now allowed to be sold as gluten-free. In addition, oats contain many healthy components (especially beta-glucans) and thus can serve as an important supplement to the patient’s daily diet. Although a very low minority of CD patients may be sensitive to oats, several CD-patient societies in Europe promote the opportunistic approach: just try, and introduce oats in your diet gradually. One of the most beloved oat products may become the gluten-free oat bread. This requires new baking technologies and recipes (Londono et al., in preparation) (Fig. 5). Currently, the first generation of pure oat bread is on the market in The Netherlands (www.broodpakket.nl)

In conclusion, a variety of alternative strategies are under development to lower the level (the burden) to the consumers of gluten in foods in general, as well as to eliminate CD-immunogenic epitopes in particular, aiming at significantly fewer and less severe cases of CD and GS.
Acknowledgements
This research was financed in part by the Celiac Disease Consortium, an Innovative Cluster approved by the Dutch Genomics Initiative (BSIK03009), and partly funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation (EL&I) (KB- 05-001-019-PRI)
References
1. Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther 2007; 26: 1217-1225.
2. Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterol 2009; 137: 88-93.
3. Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebocontrolled trial. Am J Gastroenterol 2011; 106: 508-514.
4. Junker Y, Kim SJ, Leffler D, et al. Identification of wheat alpha-amylase/trypsin inhibitors (ATIs) as triggers of innate immunity in celiac disease. Poster abstract 114 from the 14th International Coeliac Disease Symposium, Oslo 20.- 22.06.2011.
5. Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC medicine 2011; 9: 23.
6. Euromonitor (2011). A gluten-free for all drives product sales. http://www.reuters.com/article/2011/09/29/uk-food-glutenfreeidUSLNE78S00W20110929.
7. Atchison J, Head L, Gates A. Wheat as food, wheat as industrial substance; comparative geographies of transformation and mobility. Geoforum 2010; 41: 236-246.
8. Directive 2003/89/EC of the European Parliament and of the Council amending Directive 2000/13/EC as regards indication of the ingredients present in foodstuffs. Official Journal of the European Union, 25.11.2003, L 308: 15-18.
9. Van den Broeck HC, De Jong HC, Salentijn EMJ, et al. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: Wheat breeding may have contributed to increased prevalence of celiac disease. Theor Appl Genet 2010; DOI: 10.1007/s00122-010-1408-4.
10. Van den Broeck HC, Chen HB, Lacaze X, et al. In search of tetraploid wheat accessions reduced in celiac disease-related gluten epitopes. Mol Bio Syst 2010; 6: 2206-2213.
11. Loponen J. Prolamin degradation in sourdoughs. In: Academic dissertation. Helsinki 2006; ISBN 925-10-3582-X (pdf).
12. Greco L, Gobbetti M, Auricchio R, et al. Safety for patients with celiac disease of baking goods made of wheat flour hydrolysed during food processing. Clin Gatroenterol Hepatol 2011; 9: 24-29.
13. Mitea C, Salentijn EMJ, van Veelen P, et al. A universal approach to eliminate antigenic properties of alpha-gliadin peptides in celiac disease. PLoS ONE 2010; 5: e15637.
14. Koning F, Smulders MJM. Gluten toxicity, how to get rid of it. In: Proceedings of the 24th Meeting of the Working Group on Prolamin Analysis and Toxicity 2011; ASBN: 978-3-942267-18-2, pp. 63-67.
15. Commission Regulation EC 41/2009 of 20 January 2009 concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten. Official Journal of the European Union, 21.1.2009, L 16: 3-5.
Detection of toxic fragments from gluten
using a new monoclonal antibody-based test
Elisabeth Halbmayr-Jech1, Elisabeth Hammer1, Richard Fielder2, Jacqueline Coutts2, Adrian Rogers2
1 Romer Labs Division Holding, Technopark 1, A-3430 Tulln, Austria
2 Romer Labs UK Ltd, The Heath Business and Technical Park Runcorn, Cheshire WA7 4QX, United Kingdom
Introduction
Over the past decade the demand for food intolerance food products has soared, particularly, in the USA. There the number of diagnosed sufferers of coeliac disease (CD) still remains low (around 1 %) whereas the spectrum of consumers who have difficulty in digesting gluten has grown to around 10 %. These individuals show varying degrees of gluten sensitivity (GS) but show an improvement when following a gluten-free diet. Furthermore, there is a growing perception amongst increasing numbers of consumers that a gluten-free diet is better for you.
In conjunction with these changes in the marketplace for such foods there has been a better clinical understanding of the causes of CD and more recently GS. This understanding is helping to change the analysis of gluten, which currently relies on the R5 Mendez ELISA for many product assertions of a gluten-free status. New developments in gluten analysis are moving away from the concept of “gluten detection” towards the direction of “indicating the potential toxicity of gluten” for the safety of CD and GS food consumers. For this reason, the development of a new monoclonal antibody, called G12, represents an important landmark in assay method development because it reacts specifically to a protein fraction which is toxic for CD patients.
The G12 antibody specifically recognises a potent, immunotoxic fragment of a gliadin protein present in gluten. This so-called 33-mer fragment is the end-product of digestion, and has the potential to survive the enzymatic digestion processes and accumulate in the upper tract of the small intestine. It is therefore a particularly recalcitrant molecule for use as an analytical marker. The peptide structure of this 33- mer (LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF) was identified by the University of Stanford in 2002 [1]. The G12 monoclonal antibody was raised to a hexameric sequence of this fragment and demonstrates cross-reactivity to the prolamins from wheat, barley and rye but shows no cross reactivity to the safe grains maize or rice [2-4]. Additionally, its reactivity to the prolamin of oats may aid the discussion concerning the safe inclusion of oats in the diet of CD patients and the presence or absence of gluten. The G12 antibody may shed light on this debate due to ongoing work looking at its specificity to potentially immunotoxic sequences present in oats [5,6]. Due to all these potential benefits offered by this new approach using the G12 antibody and impending legislation for the labelling of gluten-free foods in Europe and the US, two quite different G12-based test kit methods have been developed. Firstly, a lateral flow device called AgraStrip® Gluten G12 for qualitative screening in the factory has been developed with the flexibility to set the cut-off limit to one of three levels (5, 10 or 20 ppm gluten). Secondly, a sandwich ELISA called AgraQuant® Gluten G12 for laboratory quantitation has also been developed, for which results from internal validation studies are reported.
Material and methods
Test Kit: The AgraQuant® Gluten G12 (COKAL0200) is a 96 well Sandwich ELISA test kit which includes following items: Package Insert, Certificate of Performance, 5 standards (0, 4, 20, 80, 200 ppm gluten) calibrated against PWG-gliadin.
PWG gliadin, Gluten G12 antibody coated microwells, ready-to-use extraction solution, 5x concentrated dilution buffer, 10x concentrated wash buffer, ready-to-use conjugate, ready-to-use substrate, ready-to-use stop solution and 1 sachet of fish gelatin.
Methodology: From a 5 g of homogenised sample, a 0.25 g portion is taken and added to 2.5 mL of extraction buffer and mixed well. The extract is incubated at 50 oC for 40 min, allowed to cool before adding 80 % ethanol and mixing well. Extracts are then shaken for one hour at room temperature using a rotator. The extracts are centrifuged at 2000 g to obtain a clear aqueous layer (filtered if necessary) and the supernatant diluted 1:10 with pre-diluted sample dilution buffer. The sample extract is then ready for addition to an ELISA transfer plate. Using a single channel pipette add 150 L of each ready-to-use standard or prepared sample into the appropriate well. Using a multichannel pipette transfer 100 L of each ready-to-use standard or prepared samples into the corresponding antibody coated microwells. Add 100 L of each ready-to-use standard or prepared sample into the appropriate well and incubate for 20 min at room temperature. Wash the plate 5 times and dry. Using an 8-channel pipette, dispense 100 L of conjugate into each well and incubate for 20 min at room temperature. Wash the plate 5 times and dry. Pipette 100 L of the substrate into each microwell using an 8-channel pipettor. Incubate at room temperature for 20 min. Pipette 100 L of stop solution into each microwell using an 8-channel pipettor. Read the strips with a microwell reader using a 450 nm filter.
Calibration: The kit standards were created using vital wheat gluten (Roquette, UK), which was extracted according to the kits’ sample extraction method. Taking into account the dilution during sample preparation the concentrations corresponded to 4, 20, 80, 200 ppm. A set of standard solutions with 5 ng/mL, 25 ng/mL, 100 ng/mL and 250 ng/mL gliadin using PWG-gliadin were also prepared. Assuming that gluten concentration is twice the gliadin concentration, the set of standards was used to calibrate the vital wheat gluten extract. This was achieved by making a serial dilution of the vital wheat gluten extract in dilution buffer and running the G12 ELISA using PWG-gliadin as the standards. The vital wheat gluten extract could then be diluted using the dilution buffer to a final concentration of 10 ng/mL, 50 ng/mL, 200 ng/mL and 500 ng/mL gluten. The dilution buffer was used as a blank. Both sets of standards were run in six replicates on the G12 sandwich assay.
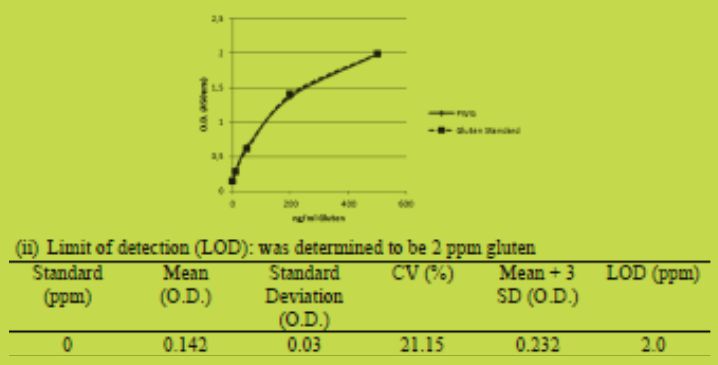
Limit of detection: the assay response of 47 buffer blank replicates was determined across 10 assays, the mean plus three standard deviations was calculated to determine the limit of detection.
Cross-reactivity: pure, uncontaminated samples of commodities were analysed in duplicate to assess the response in the assay.
Spiked commodities: extracts of vital wheat gluten were added to test portions of the samples to provide levels of 5 ppm and 20 ppm gluten in the sample, before the samples were extracted by the standard procedure.
Proficiency data: the performance of the AgraQuant® Gluten G12 ELISA was compared to the Ridascreen® Gliadin kit (Item Number R7001 from R-Biopharm), an R5 Mendez ELISA test kit. The performance was assessed by analysis of proficiency samples obtained from previous FAPAS rounds. FAPAS round 2781 consisted of a testing material prepared using a gluten and wheat free chocolate cake mix. Test materials B and C were created with the addition of a gluten and wheat containing chocolate cake mix. Test material A was prepared solely from the gluten- and wheatfree chocolate cake mix.
Results
AgraQuant® Gluten G12
(i) Calibration: the gluten standards in the kit were found to be closely aligned to the PWG-standard.
(iii) Cross-reactivity: The AgraQuant® Gluten G12 kit when tested against an extensive panel of seeds, nuts, starches, oils, naturally gluten-free foods and miscellaneous samples (including herbs and pulses) gave responses below the lower limit of quantitation of 4 ppm gluten whereas control materials (such as wheat flour) gave a positive response (above the upper limit of quantitation) as expected.
(iv) Spiked commodities: with a range of spiked samples at 10 ppm the AgraQuant® Gluten G12 gave recoveries in the range 96 - 140 %
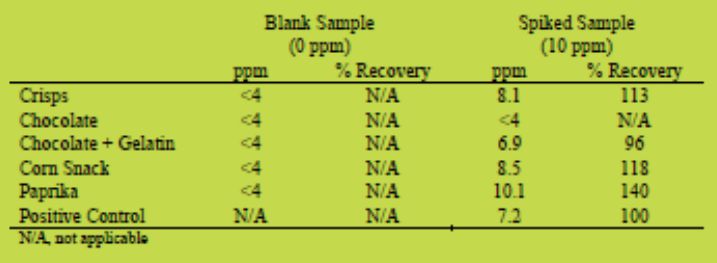
(v) Proficiency data: the AgraQuant® Gluten G12 performed similar to the Ridascreen® Gliadin ELISA in the validation experiments with proficiency samples providing responses close to expected results

Conclusion
Results have demonstrated that the AgraQuant® Gluten G12, a sandwich ELISA using the monoclonal G12 antibody, gave results for gluten analysis close to those expected across a range of samples. The R5 Mendez ELISA, of which the Ridascreen® kit used in this study is an example, is the current Type 1 method recommended by the internationally agreed Codex Standard 118:1979. The extraction of samples, the calibration of the test kit and the ELISA procedure of both the AgraQuant® and Ridascreen® kits evaluated are broadly similar. Where differences in results occur (with the samples tested) a possible explanation includes the difference in the specificity and sensitivity of the R5 and G12 antibodies. G12 detects the 33-mer from α2-gliadin which has been identified as one contributor to gluten immunotoxicity. The high correlation between the presence of the peptide and the amount of cereal that is toxic to coeliac disease patients should provide a better indication of the safety of foods than is currently possible with the R5-based assays. This is in close agreement with one of the main provisions for gluten methods in the current Codex Standard that: the antibody used should react with the cereal protein fractions that are toxic for persons intolerant to gluten and should not cross-react with other cereal proteins or other constituents of the foods or ingredients. Furthermore, the reactivity of G12 with oats potentially correlates with the immunotoxicity of these dietary grains though this is a highly contentious subject and one where further clinical and analytical investigation is needed. Therefore, the G12 Sandwich ELISA is a very attractive candidate method for the support of gluten-free labelling. This comes at a time when regulatory thresholds are being introduced for gluten-free foods in both Europe on January 1, 2012 (EC Regulation 41/2009), even though analytical methods are not being specified; and also in the US where draft proposals for labelling regulations are being discussed. To understand more about the potential offered by the AgraQuant® Gluten G12, further studies are needed to quantify the relative reactivity to cereals, the performance of the antibody with respect to oats and a performance assessment of the test kit method through international collaborative ring-trials. This data should provide evidence of a further advancement in gluten analysis that will allow more widespread acceptance of the method in order to support both compliance with new labelling legislation and the safety of such foods.
References
1. Shan L, Molberg Ø, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science 2002; 297: 2275-2279.
2. Ehren J, Govindarajan S, Morón B, et al. Protein engineering of improved prolyl endopeptidases for celiac sprue therapy. Protein Eng Des Sel 2008; 21: 699-707.
3. Morón B, Bethune MT, Comino I, et al. Toward the assessment of food toxicity for celiac patients: characterization of monoclonal antibodies to a main immunogenic gluten peptide. PLoS ONE 2008; 3: e2294.
4. Morón B, Cebolla A, Manyani H, et al. Sensitive detection of cereal fractions that are toxic to celiac disease patients by using monoclonal antibodies to a main immunogenic wheat peptide. Am J Clin Nutr 2008; 87: 405-414.
5. Rocher A, Colilla F, Ortiz ML, et al. Identification of the three major coeliac immunoreactive proteins and one alpha-amylase inhibitor from oat endosperm. FEBS Lett 1992; 310: 37-40.
6. Arentz-Hansen H, Fleckenstein B, Molberg Ø, et al. The Molecular Basis for Oat Intolerance in Patients with Celiac Disease. PLoS Medicine 2004; 1: 84-92.
Analysis of oat and buckwheat proteins by sandwich R5 ELISA
Päivi Kanerva, Outi Brinck, Hannu Salovaara, Tuula Sontag-Strohm
University of Helsinki, Finland, Department of Food and Environmental Sciences,
Faculty of Agriculture and Forestry
Introduction
Products containing oats or buckwheat can be included in gluten-free diet. Buckwheat, as being a pseudocereal, has always been considered safe for coeliac patients. Despite of several clinical studies that have shown the safety of oats, oats still have a contradictory status and the recommendations involving oats in a gluten-free diet differs between countries. The Codex standard for food for special dietary use for persons intolerant to gluten states that oats may be included in a gluten-free diet if their purity from wheat, rye and barley has been checked [1]. The EU Commission has adopted this, and oats that are free from contamination are consequently allowed for people with coeliac disease in the countries of the EU. Several studies carried out on oats including long-term trials have proven their suitability for coeliac patients [2-6].
Oats have been a part of the gluten-free diet in Finland for about 15 years without any cases of clear symptoms of coeliac disease. About 86 % of Finnish coeliac patients use oats in their diet. Oats offer a good addition to the gluten-free diet. Also, the overall gluten intake may be reduced by having oats in a gluten-free diet [7]. The main problem with oats is their contamination with wheat and barley. A special production chain has been established in Finland for oat-based products intended for gluten-free market that ensures that they cannot be contaminated during any step of the production line.
Protein compositions of oats and buckwheat are different from cereals that are considered harmful for coeliacs, i.e. wheat, barley and rye. Prolamins constitute the major fraction in wheat, barley and rye, but in oats and buckwheat, salt-soluble globulins are more dominant. This characteristic is similar to protein composition of legumes. Prolamin fraction in oats is about 10 % of the total protein content and in buckwheat less than 4 %. Oat prolamins have monomeric nature and about 10 % of their amino acid content is proline which is about a half of the amount that is found from the prolamins of harmful cereals. Buckwheat proteins were reported to contain less than 5 % of proline [8].
Materials and methods
Five oat samples were selected from products that had been specially produced, prepared and processed in a way to avoid contamination. The samples included seeds with hulls, heated dehulled seeds, oat flakes, oat flour and oat bran. The samples were extracted with 60 % ethanol, 40 % 1-propanol or the cocktail solution. Lichenase enzyme was added to decrease the viscosity caused by oat β-glucan. Milk powder was added to adsorb disturbing polyphenols. The samples were analysed by sandwich R5 ELISA according to the instructions of the manufacturer (R7001 Ridascreen Gliadin, R-Biopharm, Darmstadt, Germany).
Similarly five buckwheat samples were selected. The samples included seeds, heated seeds, flour, flavoured flour and macaroni. The samples were extracted before sandwich R5 ELISA analysis with the cocktail solution in the presence of milk powder.
Protein contents were analysed using a Dumas combustion method (Vario MAX CN, Germany) with N x 6.25.
Results and discussion
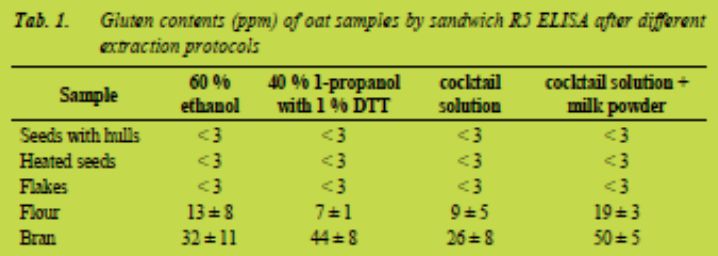
Oat seeds, heated oat seeds and oat flakes were all tested negative by sandwich R5 ELISA. However, oat flour and oat bran gave higher results for gluten content than what was expected. Quantified gluten content of oat flour after extraction with different extraction protocols stayed below the limit of 20 ppm that has been set for gluten-free products (Tab. 1). However, quantified gluten content of oat bran exceeded the limit after all extraction protocols.
Since the possibility of contamination by harmful cereals was excluded, we studied protein and polyphenol content of oat bran and oat flour, and compared them to the other oat samples. No clear difference was observed in phenolics. The total polyphenol content of oat bran was somewhat higher than in other samples, but the total polyphenol content of oat flour was the smallest of all tested samples.
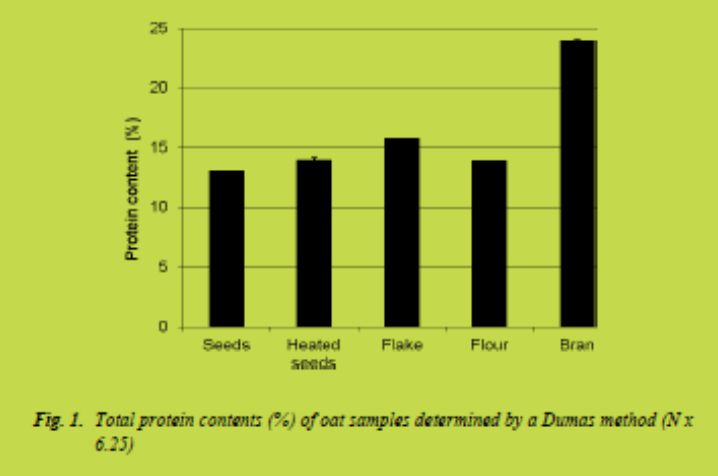
Clearer difference was, however, observed in the total protein contents of the samples
(Fig. 1). The total protein content of the bran was significantly higher than in other samples. This suggests that R5 antibody reacts with oat proteins and in the presence of high amounts of protein, the method based on R5 may lead to the high gluten measurements.
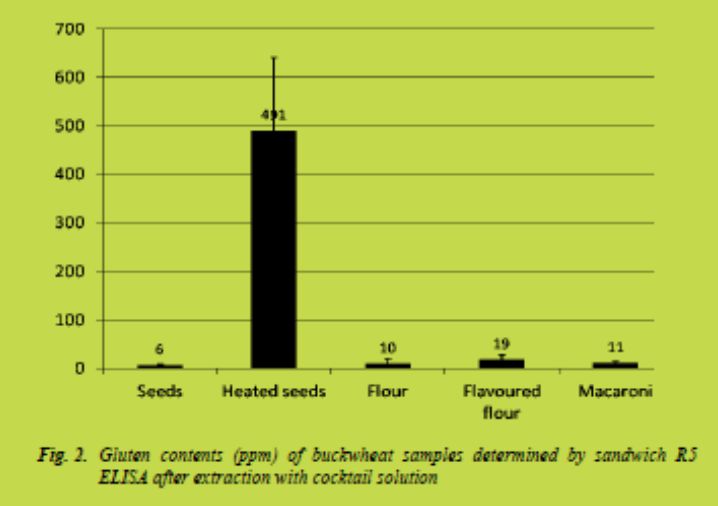
The buckwheat sample made of heated buckwheat seeds gave unexpectedly high gluten content when measured by sandwich R5 ELISA. The results obtained for a freshly milled sample were up to 400 ppm (Fig. 2). However, decrease in the amount of gluten measured in heated buckwheat was noticed over time. Heating may change the structure of buckwheat proteins which makes them able to react with the antibody. These changes seem to be reversible to some extent.
Conclusions
Unnecessary exclusion of pure fibre rich oat bran products from the selection of gluten-free foods has occurred due to the high gluten levels measured from oat bran.
The gluten levels exceeded the limit of 20 ppm, which has been set for the gluten-free oat products. Also labelling of some of the products made of buckwheat as gluten-free may be endangered, because of the heat-induced changes in buckwheat proteins.
References
1. Codex Stan 118-1979. The Codex Standard for foods for special dietary use for persons intolerant to gluten. Obtained from http://www.codexalimentarius.net, August 30th 2011.
2. Janatuinen EK, Pikkarainen PH, Kemppainen TA, et al. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med 1995; 333: 1033-1037.
3. Janatuinen EK, Kemppainen TA, Pikkarainen PH, et al. Lack of cellular and humoral immunological.responses to oats in adults with coeliac disease. Gut 2000; 46: 327-331.
4. Picarelli A, Di Tola M, Sabbatella L, et al. Immunologic evidence of no harmful effect of oats in celiac disease. Am J Clin Nutr 2001; 74: 137-140.
5. Holm K, Mäkki M, Vuolteenaho N, et al. Oats in the treatment of childhood coeliac disease: a 2-year controlled trial and a long-term clinical follow-up study. Aliment Pharmacol Ther 2006; 23: 1463-1472.
6. Kemppainen T, Janatuinen E, Holm K, et al. No observed local immunological response at cell level after five years of oats in adult coeliac disease. Scand J Gastroenterol 2007; 42: 54-59.
7. Løvik A, Gjøen AU, Mørkrid L, et al. Oats in a strictly gluten-free diet is associated with decreased gluten intake and increased serum bilirubin. Eur J Clin Nutr Metabol 2009; 4: e315-e320.
8. Javornik B, Kreft I. Characterization of buckwheat proteins. Fagopyrum 1984; 4: 30-38.
Gluten and nutritional content in a “gluten-free” diet composed according to the Swedish nutritional recommendations
Karolina Biel, Heléne Enghardt-Barbieri, Ylva Sjögren-Bolin
National Food Agency, Uppsala, Sweden
Introduction
Exposure to gluten-containing grains, e.g. wheat, rye and barley, causes immune cellmediated damage to the lining of the small intestine in patients with coeliac disease [1]. According to a review, the limit for a tolerable daily intake of gluten in coeliac patients is somewhere between 10 - 100 mg gluten [2]. A double-blind placebocontrolled trial showed that the daily intake of gluten in coeliac patients should be less than 50 mg [3]. According to the Commission regulation (EC) No 41/2009 foods can be labelled “gluten-free” and “very low gluten” if they fulfill certain criteria and the gluten content does not exceed 20 mg gluten/kg and 100 mg gluten/kg, respectively.
The damage of the proximal small intestine leads to malabsorption of certain nutrients e.g. folate, iron and calcium [4]. Avoidance of gluten-containing foods results in serologic and histological remission as well as improved nutritional status and growth for the majority of patients. Still, studies report that the “gluten-free” diet might not be nutritionally adequate [5-7]. Coeliac patients consumed lower amounts of folate compared to controls and these values were well below the recommendations according to a Swedish study [5]. In addition, the majority of gluten-free foods, in the US, contained lower amounts of folate and iron compared to their gluten-containing counterparts [6]. Also, several products contained lower amounts of fiber.
Additionally, less than 50 % of female coeliac patients consumed recommended amounts of fiber, iron and calcium [7]. In 2003 the Swedish National Food Agency published the report Swedish Nutrition Recommendations Objectified (SNO) [8]. The SNO report constituted the nutritional recommendations translated into separate daily menus for four weeks for the two reference persons Hans and Greta. Their diets were composed in order to fulfill the nutritional requirements, without exceeding the energy levels, and still to function according to Swedish dietary habits.
The aims of this study were to investigate which amounts of gluten the SNO menus contain, when gluten-containing foods were replaced by “gluten-free” foods, and to compare whether the fat, protein, carbohydrate, iron, folate and fiber contents in the “gluten-free” SNO menus differ from the gluten-containing ones.
Methods
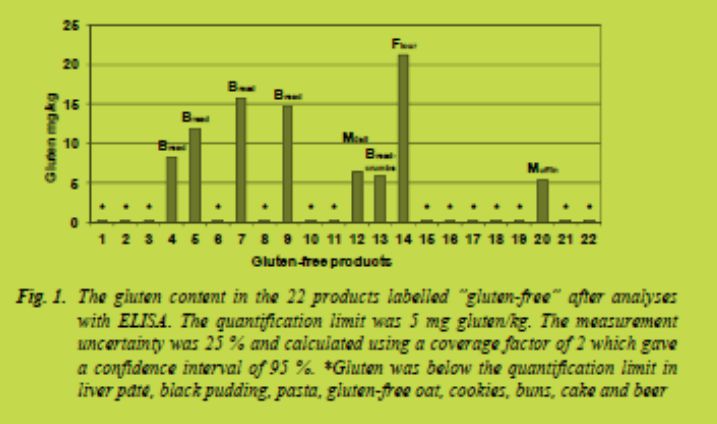
Twenty-two products labelled “gluten-free” were chosen in order to replace the glutencontaining foods in the SNO menus. Examples of replaced food categories are pasta, bread, grains, cookies, flour, black pudding and liver pâté (Fig. 1). In addition, quinoa substituted couscous. The chosen products mainly represented the most often sold “gluten-free” products on the Swedish market according to the Nielsen index 2009.
The products were chosen in order to be comparable to the “gluten-containing” food in the original SNO menus e.g. rye bread rich in fiber was exchanged for “gluten-free” bread rich in fiber. Replacements were made 275 times in total. Sausages and similar products were not replaced as they can be produced without wheat, rye and barley.
The gluten content in the twenty-two products labelled “gluten-free” was analysed with the R5 Sandwich ELISA (RIDASCREEN®, Gliadin, R-Biopharm, Damstadt, Germany). The result of the gluten content is shown in Fig. 1. Eight products contained gluten above the quantification limit (5 mg gluten/kg). These values (5 - 21 mg gluten/kg) were used in the calculations of the gluten content in the menus. Gluten was below the quantification limit in the remaining fourteen products labelled “glutenfree”.
The gluten content of these products was set to zero in the calculations.
The content of energy, protein, fat and carbohydrates were read from the labelling on the package. Iron, folate and fiber content could sometimes be read from the package.
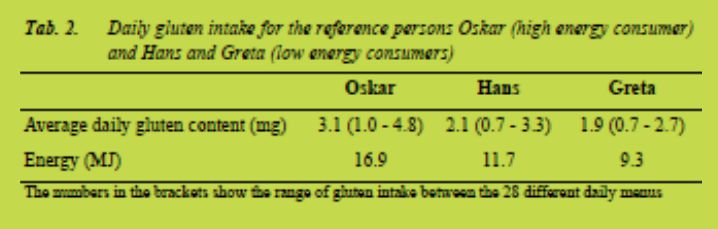
In other instances the companies were asked for these data. The male and female reference persons represent two average Swedes with little physical activity and thus low energy intake, Hans 11.5 MJ and Greta 9.1 MJ. In addition to Hans and Greta a young (25 - 34 years) male reference person, with high levels of physical activity, was included and named Oskar. Oskar consumed 16.9 MJ and the gluten consumption by Oskar was calculated with a factor of 1.47 from the gluten content in Hans’ menu.
In total, 28 different daily menus for Hans and Greta are described in the SNO report. An example of one day, Friday week 1, in Greta´s original SNO menu is shown in Tab. 1. The table also shows Friday week 1 when gluten-containing foods have been exchanged for “gluten-free” foods. The menus of Hans and Greta, for all 28 days, were placed into two separate Excel files. The nutrient data regarding all foods that do not contain gluten (e.g. fruit, vegetables, meat and milk) were kept and the gluten content was set to zero. The portion sizes of the “gluten-free” products were adjusted to be similar to the original portion sizes i.e. one piece of bread was replaced by one piece of “gluten-free” bread. The gluten content and the nutritional content of the “gluten-free” products were added and calculations were performed.

Results and discussion
Gluten
The average daily gluten content in the menus of Oskar, Hans and Greta is shown in Tab. 2. Oskar consumed most energy and thus most gluten. His average daily gluten intake was 3.1 mg gluten/day. The daily menu that contained the highest amount of gluten contained 4.8 mg gluten. This is well below the tolerable daily intake of gluten at 10 - 50 mg gluten/day [2,3]. Yet, the measurement uncertainty of the method is 25 % and a gluten concentration below 5 mg/kg could not be quantified meaning that the
average intake of gluten might be slightly higher, although below 10 mg gluten/day.
In average Oskar consumed 626 g of “gluten-free” products daily. Bread, buns and cookies constituted the largest proportion. Pasta was in average consumed 2.5 times/week. The total amount, 626 g “gluten-free” products, was used to calculate which amounts of gluten Oskar would consume daily if the products would contain concentrations of gluten at the thresholds for products labelled “gluten-free” and “very low gluten” i.e. 20 mg gluten/kg and 100 mg gluten/kg, respectively. If all “glutenfree” products would contain 20 mg gluten/kg Oskar would consume 12.5 mg gluten/day. If Oskar only ate products labelled “very low gluten” and they all contained 100 mg gluten/kg he would consume 63 mg gluten/day. This is above the daily tolerable intake [2,3]. Still, most products on the Swedish market are labelled “gluten-free”, meaning that they contain less than 20 mg gluten/kg. In addition, the SNO menus contain almost twice the amount of bread as the average Swedish diet, according to a national diet survey performed 1997 - 1998 [9]. The calculations thus show that it is not very likely that even a high energy consumer, who consumes products labelled “gluten-free”, will consume harmful amounts of gluten. Mislabeling, contamination and low knowledge at restaurants are more likely to lead to consumption of foods that are harmful for patients with coeliac disease.
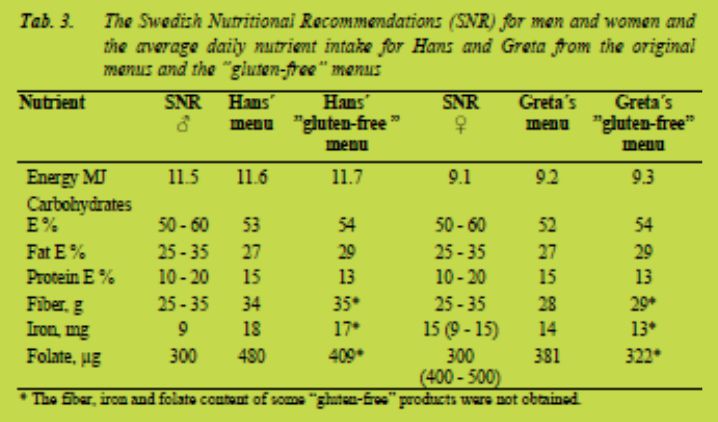
Nutrients
Tab. 3 shows the Swedish Nutritional Recommendations (SNR) and the average nutrient intake in the SNO menus (Hans and Greta) as well as in the “gluten-free” SNO menus. The energy content was 0.2 MJ higher in the “gluten-free” SNO menus compared to the recommendations. This was partly due to the adjustment of portion sizes i.e. that one piece of bread was replaced by one piece of “gluten-free” bread and not exactly the same weight of bread. Even though the energy percent of protein was somewhat lower and the energy percent of fat and carbohydrates were somewhat higher in the “gluten-free” menus, compared to the original SNO menus, all these macronutrients were within the recommended ranges.
Interestingly, the fiber content of the “gluten-free” menus was within the recommended ranges (Tab. 3) even though the fiber content of four “gluten-free” products was set to zero as these values were not obtained. Foods rich in fiber that did not contain gluten, e.g. fruit and vegetables, accounted for 51 and 52 % of the fiber intake in the menus of Hans and Greta, respectively. Adequate fiber content can thus be reached for coeliac patients as long as they eat “gluten-free” products rich in fiber and the recommended amounts of fruit and vegetables (500 g/day). Unfortunately, only 5 - 10 % of Swedish women reach the fiber recommendations according to the national diet survey [9]. Previously it was shown that 50 % of coeliac patients did not reach the recommended amounts of fiber [7]. This is probably due to a diet low in fiber in general and not to the “gluten-free diet” per se. The intake of oatmeal porridge and hard bread made of oats in the diets is most likely contributing to the fiber content.
Oats has been shown to increase the nutritional content in the “gluten-free” diet [10]. The iron content was obtained for fourteen of the twenty-two “gluten-free” products.
Hans who consumed more food, reached the recommendations even in the “glutenfree” diet (Tab. 3). The recommended daily intake of iron for fertile females is 15 mg iron meaning that the “gluten-free” diet of Greta, which contained 13 mg iron, did not reach the recommendations. Not even Greta´s original SNO menu reached the recommendations for fertile women. Fertile women who are low energy consumers might have problems in reaching the recommended amounts of iron, regardless of whether they are coeliac patients or not. Foods rich in iron that do not contain gluten accounted for 52 and 54 % of the iron intake in the diets of Hans and Greta, respectively.
The folate content was obtained for nine of the twenty-two “gluten-free” products. Still, Hans who consumed more food reached the recommendations even in the “gluten-free” diet (Tab. 3). Food rich in folate that did not contain gluten, e.g. fruits, vegetables and legumes, accounted for 79 % of the folate intake in the diets of Hans and Greta. The recommended daily folate intake for fertile females is 400 μg folate which means that Greta´s “gluten-free” diet did not reach the recommendations. Not even Greta´s original SNO menu reached the recommendations. Fertile women who are low energy consumers might have problems in reaching the recommended amounts of folate, regardless of whether they are coeliac patients or not. Nutritional recommendations are calculated to meet the needs of the healthy population with the highest needs. Coeliac patients might be among the ones whose nutritional status is lower and it might thus be especially important for them to reach the recommended levels. In Sweden women who are planning to become pregnant are recommended to take folate supplements [11].
Conclusions
The gluten intake from the menus was well below the indicated tolerable threshold of 10 - 50 mg gluten/day, even for the high energy consumer who consumes larger amounts of “gluten-free” bread and similar products. Fertile women who are low energy consumers might need to consider their intake of iron and folate, regardless of whether they have coeliac disease or not. Recommended daily intakes of fiber can be reached by coeliac patients as long as they consume recommended amounts of fruits and vegetables and “gluten-free” products rich in fiber.
References
1. Di Sabatino A, Corazza GR. Coeliac disease. Lancet 2009; 373: 1480-1493. 2. Hischenhuber C, Crevel R, Jarry B, et al. Review article: safe amounts of gluten for patients with wheat allergy or coeliac disease. Aliment Pharmacol Ther 2006; 23: 559-575.
3. Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebocontrolled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007; 85: 160-166.
4. Niewinski MM. Advances in celiac disease and gluten-free diet. J Am Diet Assoc 2008; 108: 661-672.
5. Hallert C, Grant C, Grehn S, et al. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther 2002; 16: 1333-1339.
6. Thompson T. Folate, iron, and dietary fiber contents of the gluten-free diet. J Am Diet Assoc 2000; 100: 1389-1396.
7. Thompson T, Dennis M, Higgins LA, et al. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron,
calcium and grain foods? J Hum Nutr Diet 2005; 18: 63-69.
8. Enghardt-Barbieri H, Lindvall C. Swedish Nutrion Recommendations Objectified. National Food Agency 2003.
9. Becker W, Pearson M. Riksmaten 1997-98, Kostvanor och näringsintag i Sverige, Metod och resultatanalys: National Food Agency 2002.
10. Lee AR, Ng DL, Dave E, et al. The effect of substituting alternative grains in the diet on the nutritional profile of the gluten-free diet. J Hum Nutr Diet 2009; 22: 359-363.
11. National Food Agency website. http://www.slv.se/en-gb/Folic-acid/.
Gluten-specific peptidase activity of different cereal
species and cultivars induced by germination
Theresa Schwalb, Herbert Wieser, Peter Koehler
German Research Centre for Food Chemistry, Freising, Germany
Introduction
In order to detoxify gluten-containing raw materials and foods for coeliac patients, a number of bacterial and fungal peptidases (so-called prolyl endopeptidases) have been suggested in the last years [1,2]. Our previous studies have shown that gluten-specific peptidases can also be activated by germinating cereal grains. The enzymatic activity is primarily enriched in the bran containing both endo- and exopeptidases with the ability to cleave peptide bonds next to proline residues [3]. Distinct advantages as compared to bacterial and fungal peptidases are obvious: they have unique specifities optimised for gluten degradation by nature and are naturally safe; their production uses a well-established technological process (malt and beer production) and is simple and cheap. Furthermore, no genetic engineering is necessary. However, the dependence of peptidase activity on cereal species and cultivars is not yet known. Therefore, the aim of the work presented here was to study the peptidase activity of germinated grains from different wheat species and other cereal cultivars by using gliadin as a proteinbased substrate and a coeliac-toxic peptide from -gliadins as a peptide-based substrate.
Materials and methods
Grains from common wheat (cultivars (cvs.) Hermann and Winnetou), spelt (cvs. Franckenkorn and Oberkulmer Rotkorn), emmer (cvs. Osiris and Ramses), einkorn (cvs. FR7037 and UH36582), rye (cvs. Conduct and Guttino), barley (cvs. Conchita and Marthe), oats (cvs. Ivory and Scorpion) and maize (cvs. Grosso and Ricardinio) were obtained from different German breeding companies. Dehulled grains were germinated for seven days at 15 or 25 °C, lyophilised and milled into white flour and bran [3]. The protein composition of the different brans was characterised by means of modified Osborne fractionation [4]. The peptidases of brans were extracted with a sodium acetate buffer (0.2 mol/L, pH 4.0) at 4 °C [5]. For the determination of the activity, aliquots of the extracts were incubated at 50 °C, pH 4.0 or 6.5 for 60 min with the peptide substrate PQPQLPYPQPQLPY (one-letter-code for amino acids) purchased from GenScript Corporation (Piscataway, NJ, USA) or for 150 min with the protein substrate gliadin isolated from flour of wheat cv. Cubus according to [6].
Reactions were stopped by heating at 90 °C for 10 min. Peptide or gliadin degradation was quantified by RP-HPLC using UV absorbance at = 210 nm [4,5].
Results and discussion
Germination
Dehulled grains of the different cereals were germinated for seven days at 15 or 25 °C, freeze-dried and milled into white flour and bran. Only the latter was further investigated, because bran was shown to have higher peptidase activity than white flour [3]. The comparison of the quantitative protein composition of bran from nongerminated and geminated grains by means of modified Osborne fractionation showed that, generally, the salt-soluble albumin/globulin fractions considerably increased during germination, whereas storage proteins (prolamins, glutelins) decreased (data not shown). This was possibly an indicator for the enrichment of peptidases in the albumin/globulin fraction and for the shift of storage proteins to the salt-soluble fraction due to extensive enzymatic fragmentation. Only cereals showing a considerable degradation of storage proteins after germination were used for further investigations.
Peptidase activity
Peptidases were extracted from bran with a sodium acetate buffer under slightly acidic conditions [3,5]. Activity was demonstrated towards both intact and degraded proteins using on the one hand a gliadin fraction isolated from wheat cv. Cubus and on the other hand the synthetic peptide PQPQLPYPQPQLPY from -gliadins as substrates.
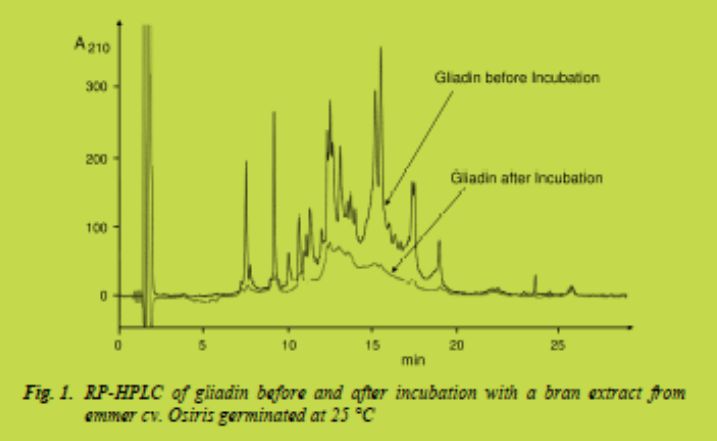
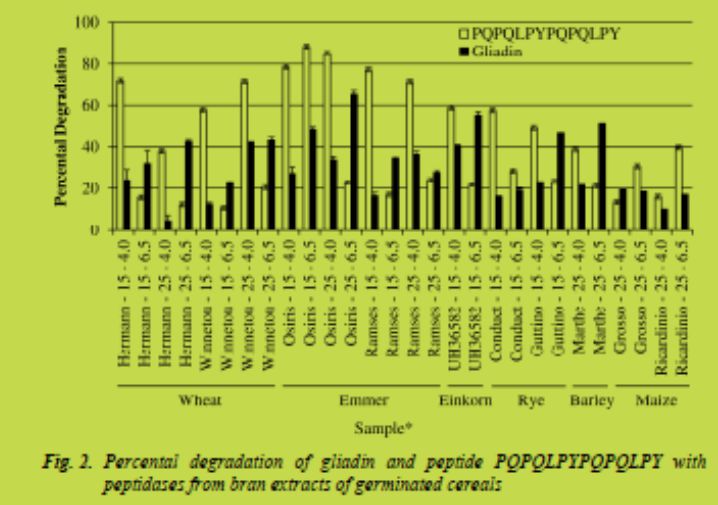
Incubations were performed at two different pH values (4.0 and 6.5), which were shown to be optimal for peptidase activity of wheat and rye bran [3]. The degradation was determined by RP-HPLC comparing peak areas at the beginning and after 60 min (peptide) or 150 min (gliadin) of incubation. Fig. 1 shows the HPLC patterns of gliadin before and after incubation with peptidases from bran of emmer cv. Osiris germinated at 25 °C. Fig. 2 gives an overview of the percental degradation of both gliadin and peptide substrates measured at pH 4.0 and 6.5. The results ranged from less than 10 % to more than 80 % degradation and were strongly dependent on cereals, cultivars, germination temperature, substrates, and incubation pH; thus, conclusions on the best conditions were not universally valid. Germinated brans of emmer cv. Osiris (germination temperature 25 °C / incubation pH 6.5), einkorn cv. UH36582 (15 °C / 6.5), and barley cv. Marthe (25 °C / 6.5) showed the highest degree of gliadin degradation. The peptide substrate was degraded most by germinated bran of emmer cv. Osiris (15 °C / 6.5 or 4.0 and 25 °C / 4.0), emmer cv. Ramses (15 °C / 4.0) and common wheat cv. Hermann (15 °C / 4.0) concerning the application.
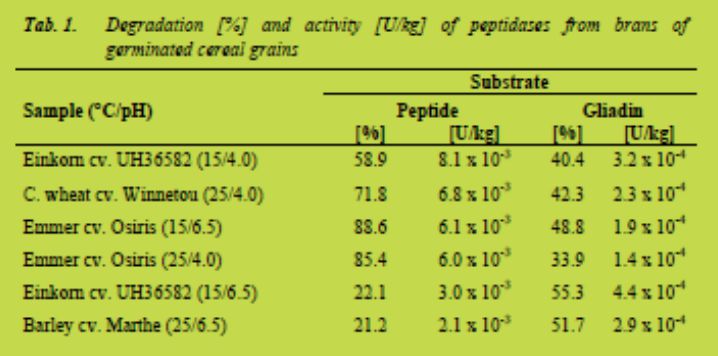
Due to economic aspects according to the application for detoxifying raw materials and foods, the activities of the cereals investigated were converted into units per kg grains. One unit is defined as the amount of enzyme that catalyses the degradation of one μmol of substrate per minute under defined conditions (here at 50 °C). Thus, the yield of the bran in relation to total dehulled grains and molecular masses of peptide (1660 g/mol) and gliadin (33716 g/mol on an average) were used for calculation. Tab. 1 summarises those cereal samples with the highest activities expressed as U/kgtowards peptide and gliadin substrates. Germinated grains from einkorn cv. UH36582, wheat cv. Winnetou, emmer cv. Osiris, and barley cv. Marthe showed the best results.
Conclusion
The present studies showed that, generally, cereal grains increase their peptidase activity towards storage proteins during germination in order to provide the growing embryo with amino acids and nitrogen. The activity, however, is strongly dependent on cereal species and cultivar, germination temperature, substrate, and incubation conditions. For the detoxification of gluten, the activity towards both intact gluten proteins and partially hydrolysed proteins is important. Therefore, a gliadin fraction of wheat flour and a toxic peptide from -gliadins were used as substrates. Both assays for measuring gluten-specific peptidase activity of brans from germinated grains were relatively simple and generated reproducible results. Thereby, the bran extracts with the highest peptidase activities could be determined and the germinated grains with the highest yield of peptidases were identified. These cereals will be used for further studies aimed at detoxifying gluten-containing raw materials and foods.
Acknowledgement
The authors wish to thank the Leibniz-Association for financial support (project no. SAW-2011-DFA-1).
References
1. Sollid LM, Khosla C. Future therapeutic options for celiac disease. Nature Clin Pract Gastroenterol Hepatol 2005; 2: 140-147.
2. Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterol 2009; 137: 1912-1933.
3. Hartmann G, Koehler P, Wieser H. Rapid degradation of gliadin peptides toxic for coeliac disease patients by proteases from germinating cereals. J Cereal Sci 2006; 44: 368-371.
4. Wieser H. Antes S, Seilmeier W. Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chem 1998; 75: 644-650.
5. Gessendorfer B, Hartmann G, Wieser H et al. Determination of celiac diseasespecific peptidase activity of germinated cereals. Eur Food Res Technol 2011; 232: 205-209.
6. Gessendorfer B, Koehler P, Wieser H. Preparation and characterization of enzymatically hydrolyzed prolamins from wheat, rye, and barley as references for the immunochemical quantitation of partially hydrolyzed gluten. Anal Bioanal Chem 2009; 395: 1721-1728.
V. Clinical research reports
The CXCR3 / CXCL10 axis. Role in coeliac disease pathogenesis
Constanza Bondar1, Romina Araya1, Ezequiel Rulli1, Luciana Guzman2, Eduardo Cueto Rua2, Nestor Chopita3, Fernando Chirdo1
1 Laboratorio de Investigación en el Sistema Inmune – LISIN, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Argentina
2 Servicio de Gastroenterología, Hospital de Niños “Sor María Ludovica”, La Plata, Argentina
3 Servicio de Gastroenterología, Hospital San Martín, La Plata, Argentina
Introduction
Active Coeliac Disease (CD) is characterised by histological changes in the intestinal mucosa, leading to an enteropathy a consequence of both innate and adaptive immunity pathogenic mechanisms. Gliadin peptides are able to induce a rapid response in the epithelia and lamina propria involving different inflammatory mediators.
Particularly, p31-43 -gliadin peptide has been the most commonly used peptide to assess the innate mechanisms elicited in the intestinal mucosa [1].
Early pathogenic events involve changes such as disruption of tight junction integrity and the production of proinflammatory cytokines, among them, IL-15 plays a major role at the initial stage. The induction of innate immunity trigger inflammatory mechanisms which could sustain the chronic process, characterised by a massive T and B lymphocyte infiltration in the intestinal mucosa of untreated patients [2].
It has been clearly established, that gliadins and glutenins peptides activate lamina propria HLA-DQ2/DQ8 restricted -CD4+ T lymphocytes. These T cells, belong to the Th1 subset, and upon activation abundantly produce IFN. Th1 cells are likely activated in mesenteric lymph nodes, circulate in the peripheral blood and then entry in the lamina propria. Migration of cells is governed by a sophisticated system of chemokines and their receptors. Different pairs of receptor/ligand determine the selective migration of lymphocytes in the intestinal mucosa under homeostatic conditions, such as MadCAM1/47 and CCL25/CCR9 [3]. However, under an inflammatory process, cell recruitment involves other pathways such as the CXCR3/ CXCL10 axis, which was pointed out as one of the most relevant promoting the arrival of cells to the inflammed tissues. This axis was involved in chronic inflammatory processes such as autoimmunity (type 1 diabetes, reumatoid arthritis) [4,5].
CXCL10 is a chemokine induced by IFN (its former name: IP-10, 10-kDa IFN- induced protein). It is also rapidly upregulated by different stimuli in inflammatory conditions. CXCL10 was detected in the serum of patients with active autoimmune diseases (such as type I diabetes and reumatoid arthritis) [6]. CXCL10 is produced by CD4+ T cells, NK and NKT cells, monocytes, dendritic cells, neutrophils, fibroblasts.
Remarkably, synoviocytes and cells, actively produce CXCL10 during the inflammatory process, arthritis or insulitis, respectively. CXCL10 interacts and activates CXCR3, receptor expressed by T lymphocytes, NK cells, eosinophils, monocytes, B lymphocytes [4]. CXCR3 is a G protein-coupled, seven-transmembrane receptor, which upon interaction with the appropriated ligand, the G protein becomes activated, causing GDP exchange for GTP. As consequence different cellular pathways are activated, i.e. calcium influx and activation of MAPK and Akt, triggering cytoskeleton rearrangement and cell movement, among other effects [4].
Consequently, CXCL10 mediates the recruitment of CXCR3+ cells. CXCR3 interacts not only with CXCL10 but also with CXCL9 and CXCL11. These chemokines are differentially express when different tissues and conditions are compared, suggesting that their biological function is not redundant [7]. Th1 cells, which are abundant in the lamina propria of untreated CD patients, express CXCR3 [8].
The aim of this work was to assess the role of the CXCL10/ CXCR3 axis in coeliac disease pathogenesis.
Patients and methods
Biopsy samples: Intestinal biopsies were taken from pediatric and adult patients suffering from different gastrointestinal symptoms on the routine procedure to diagnose coeliac disease. Diagnosis was acchieved by histological examination, serologic analysis and evaluation of clinical presentation. The present study was approved by the Ethical Committees of the HIGA San Martin and Sor Maria Ludovica Hospitals from La Plata.
Quantitative Real-Time PCR: Real-Time PCR was performed to determine the RNAm level of CXCL10, CXCR3 and IFN. Quantitative PCR was performed in iCycler Real Time PCR (BioRad). -actin as housekeeping gene was used for normalisation. Primers used are listed in Tab. I.

Multicolour fluorescence confocal microscopy: To determine the number of CXCR3+ cells in the intestinal mucosa, sections of duodenal biospies from untreated CD patients and controls were stained with anti-CXCR3 (R&D Systems; Cat: MAB160) and Alexa 488 F(ab´)2 fragment of goat anti-mouse IgG (H+L) (Invitrogen, cat A11020). To evaluate the expression of CXCL10, policlonal rabbit anti-CXCL10 (IP- 10) (Santa Cruz; Cat sc-28877) and alexa 488 goat anti-rabbit IgG (H + L) (Invitrogen, cat A11008), were used.
Images were taken in a SP5 Leica confocal microscopy, and then analysed by Image J software. Counting was performed by using Image J software properly calibrated to measure the areas. LP areas were drawn over the entire histological section and we counted positive cells over a total of 150,000 μm2 on average. Surface epithelium, villi and crypts were excluded. All countings were performed blindly by the same investigator.
Results and discussion
Gliadins/glutenins specificity and HLA-DQ2/DQ8 restriction have been well established for CD4+ T cells from intestinal lamina propria of CD patients, however, signals that sustain the Th1 pattern are still not completely known. Inflammatory factors such as: IL-15, IL-17, IL-21, IL-23 may mediate the expansion of Th1/Th17 cells in the intestinal CD mucosa. The lymphocytic infiltration in the small intestine mucosa of untreated CD patients results from both the selective recruitment of precursors and differentiated cells and the local expansion of recently activated T and B cells [1]. Since, Th1 cells express CXCR3, we sought to assess the role of CXCR3/CXCL10 axis in the recruitment of Th1 cells into the small intestinal lamina propria.
The analysis of gene expression by quantitative PCR showed that mRNA level of CXCL10 was significantly higher in intestinal biopsies of paediatric CD patients compared to healthy controls.
As expected, the mucosal samples from untreated CD patients showed a significant increase in IFN mRNA levels when compared with samples from non-CD controls.
However, contrary to previous reports [9], we did not find statistical difference in CXCR3 expression between control and coeliac paediatric samples (Fig. 1). Similar results were obtained for the adult population (not shown).
Although we could not observed a differential expression of mRNA for CXCR3 in intestinal mucosa in untreated CD patients, the immunofluorescence analysis of sections of small intestine revealed that the number of CXCR3+ cells was substantially higher in untreated CD samples compared to controls (Fig. 2).
Though we could not perform the analysis of different cell lineages expressing CXCR3, it is likely that part of CXCR3+ cells observed by confocal microscopy belong to the Th1 subset.


The analysis of CXCL10 expression by immunofluorescence confocal microscopy showed that the intestinal lamina propria of untreated CD patients contained such a high amount of CXCL10 that positives cells could not be properly counted (not shown). By confocal microscopy analysis we observed that part of the CD3+ T lymphocytes produced CXCL10 (not shown).
CXCL10 can be produced by T lymphocytes, Th17 cells, activated NK and NKT cells, macrophages, fibroblasts, endothelial cells, and epithelia. CXCL10 is overexpressed in different inflammatory conditions. For example, in reumathoid arthritis, CXCL10 was mainly expressed by infiltrating macrophage-like cells and fibroblast like synoviocytes in the synovium [10].
Similarly, herein we showed the increased production of CXCL10 in the small intestine of untreated CD patients. In addition, we observed a higher number of CXCR3+ cells in intestinal lamina propria.
Conclusion
These observations suggest that the massive production of CXCL10 in the small intestine in active CD may mediate the recruitment and activation of CXCR3+ cells. This is the first report describing the role of CXCR3/CXCL10 axis in the pathogenesis of CD.
References
1. Abadie V, Sollid LM, Barreiro LB, et al. Integration of genetic and immunolgical insights into a model of celiac disease pathogenesis. Annu Rev Immunol 2011; 29: 493-525.
2. Meresse B, Ripoche J, Heyman M, et al. Coeliac disease: from oral tolerance to itnestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol 2009; 2: 8-23.
3. Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med 2009; 9: 836-850.
4. Lacotte S, Brun S, Muller S, et al. CXCR3, inflammation and autoimmune disease. Ann NY Acad Sci 2009; 1173: 310-317.
5. Lee EY, Lee Z-E, Song YW. CXCL10 and autoimmunity disease. Autoimmun Rev 2009; 8: 379-383.
6. Shigihara T, Oikawa Y, Kanazawa Y, et al. Significance of serum CXCL10/IP-10 level in type 1 diabetes. J Autoimmun 2006; 26: 66-71.
7. Müller M, Carter S, Hofer MJ, et al. The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity. Neuropathol Appl
Neurobiol 2010; 36: 368-387.
8. Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res 2011; 317: 620- 631.
9. Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008; 135: 194-204.
10. Lee EY, Seo M, Juhnn Y-S, et al. Potential role and mechanisms of IFN inducible protein-10 on receptor activator of NFkB ligand (RANKL) expression in rheumatoid arthritis. Arthritis Res Ther 2011; 13: R104.
Treatment of type 2 refractory coeliac disease in a single centre
Suzanne C. Donnelly1, Colan Ho-Yen2, Tracey Mitchell3, Fuju Chang2, Paul J.Ciclitira1
1 Gastroenterology Department, Division of Nutritional Sciences, Kings College London
2 Pathology Department
3 Molecular Diagnostic Services, St Thomas Hospital, Westminster Bridge Road, London SE1 7EH
Abstract
Refractory coeliac disease is a rare complication of coeliac disease. It is diagnosed by persistent mucosal atrophy despite a strict gluten-free diet for at least a year, ongoing symptoms of diarrhoea and/ or evidence of malabsorption. The condition can be differentiated into two types depending on the presence or relative absence of aberrant T cells. The prognosis in type 2 refractory coeliac disease, with aberrant T cells, is guarded due to a high percentage of patients developing either nutritional complications with sepsis or enteropathy associated lymphoma. Treatment options vary due to a paucity of randomised control trials. The aim of this paper was to present a single centre’s experience in the treatment of type 2 refractory coeliac disease. In this retrospective study all cases of type 2 refractory coeliac disease diagnosed in St Thomas Hospital, London using the coeliac patient database were followed. Case notes, biological and histological data were reviewed for patients with a diagnosis of type 2 refractory coeliac disease diagnosed between 2000 and 2010. All patients were treated with prednisolone, 20 mg, and azathioprine, 2 mg/kg/day with repeat small bowel biopsy and T cell receptor analysis by PCR at 4 monthly intervals. In this report, seven out of ten patients with type 2 refractory coeliac disease were successfully treated with prednisolone and azathioprine to become either type 1 refractory coeliac disease, in 5 patients, or coeliac disease, in 2 patients, with a better 5 year survival. None of the type 2 refractory patients developed lymphoma on this treatment. Thus, prednisolone combined with azathioprine can be used successfully to treat type 2 refractory coeliac disease.
Introduction
Coeliac disease is a gluten sensitive enteropathy that affects approximately 1 % of the Northern European and American populations. Treatment comprises a lifelong glutenfree diet. A rare complication is refractory coeliac disease (RCD), when clinical symptoms and histological changes persist or recur after a good response to a gluten free diet after other causes of villous atrophy having been excluded.
Refractory coeliac disease can be classified according to the immunophenotype of intra-epithelial lymphocytes. Abnormal clonal lymphocytes with loss of surface markers CD8 and CD3 occur in type 2 RCD [1] whereas in type I RCD the majority of lymphocytes have normal surface markers and T cell receptors are polyclonal.
Differentiation between the different types of refractory coeliac disease is important as the reported 5 year survival rate varies between 40 - 58 % for type 2 RCD [2-5], and 93 % in type 1 RCD [3]. The main cause of death in type 2 RCD is progression to an enteropathy associated lymphoma (EATL) with a five year survival rate of 8 - 20 % [2,6] as well as progressive malnutrition.
Refractory coeliac disease is rare such that the evidence for the treatment is largely based on case reports, open-label observational, prospective experience and expert opinion [7-9]. It is generally accepted that prednisolone and azathioprine, 2 mg/kg/day, is the treatment of choice for type 1 RCD [10] and that an elemental diet [11] in the case of type 1 RCD or infliximab infusions [12] for type 2 RCD successfully treat a small number of patients in case reports only. Previously reported treatment regimes for type 2 RCD are under debate with previous proposals including treatment with cyclosporin [13], cladribine [13,14], fludarabine and stem cell transplantation [15,16], the latter three of which are associated with significant mortality. Prednisolone appears to correlate with improved clinical response. However, mucosal recovery is not always seen and progression to EATL has been reported not to be prevented [2,3,5]. Clinical and histological improvement was reported in 61 % of patients with type 2 RCD treated with a prolonged course of oral cyclosporin, 5 mg/kg/day, [13]. Patients who failed on cyclosporin therapy have also been treated with either intravenous cladribine, 1 mg/kg/day for 5 days, with some success or fludarabine and autologous stem cell transplantation [14,15]. There was clinical improvement with cladribine in 39 % and histological improvement in 59 % with significant improvement in the number of clonal intra-epithelial lymphocytes. However, 41 % still progressed to develop EATL with resultant mortality [15]. High dose chemotherapy with fludarabine and autologous stem cell transplantation is currently being explored as a treatment option for patients with type 2 RCD. However, the results to date provide evidence of progression to EATL [15] despite previous treatment with cladribine [16].
Methods and results
Patients with RCD were selected from St Thomas Hospital’s coeliac database using clinic letters from 2000 - 2010. All our patients with type 2 RCD (n=10) presented with diarrhoea and weight loss on clinical review. They underwent basic biochemistry and haematology evaluation (full blood count, ferritin, haematinics, HLA class II status, coeliac serology (IgA anti-gliadin antibody, IgA tissue transglutaminase antibody and anti-endomysial antibody) urea and electrolytes and liver function tests).
We undertook dietary assessment by a fully trained gastrointestinal specialist dietician, we also undertook lactulose and lactose breath tests as well as endoscopy with distal duodenal biopsies. We carried out T cell receptor characterisation by polymerase chain reaction to look for evidence of T cell receptor monoclonality at the beta and gamma T cell receptor if the biopsy had evidence of villous atrophy, Marsh 3. Tab. 1 demonstrates the patient characteristics of the ten patients diagnosed in our centre between 2000 and 2010 with type 2 refractory coeliac disease.
All patients were started on prednisolone, 20 mg, and azathioprine, 2 mg/kg/day, and followed up with repeat small bowel biopsy for histology, immunohistochemistry and PCR for T cell receptor at 4 monthly intervals. Their follow up data is presented in Tab. 2. Patients 3 and 9 have reverted from RCD to gluten-free diet responsive CD while in 5 other patients their histology has improved. The T cell receptor status has changed from clonal in 7 patients to polyclonal after treatment and in 5 of these patients the surface markings of the IELs have returned. All ten patients are still alive with no complications from their RCD or treatment with azathioprine and prednisolone.
We have experienced no problems and a good outcome with our current treatment regime of type 2 refractory coeliac disease using prednisolone and azathioprine. We present our retrospective analysis for the successful treatment of 7 out of 10 type 2 refractory coeliac disease patients using this therapeutic regime. Interestingly, Goerres [10] treated a patient with type 1 RCD with weak clonality of the T cell gamma receptor which became polyclonal after a year’s treatment with prednisolone and azathioprine.
Discussion
Prednisolone and azathioprine is a viable treatment option for patients with type 2 refractory coeliac disease. These medicines have better safety records compared to the more potent treatment options available, including the cytotoxic agents cladribine and fludarabine, which are only licensed in the UK for treatment by certified oncologists in recognised oncology centres. Our patients were treated early without developing the lower haemoglobin and albumin levels associated with poorer disease outcomes and may have benefitted for this reason. Earlier diagnosis of refractory patients may have influenced the disease outcome, although the pathogenesis of refractory coeliac disease remains poorly understood. Better understanding of the disease mechanisms may play a key role in future treatment strategies.
Individuals with coeliac disease invariably carry either the HLA-DQ2 or HLA-DQ8 haplotype with the majority from Northern Europe being HLA-DQ2 positive. We noted that in the patient cohort there is a higher than expected HLA-DQ8 population of patients. HLA-DQ is known to responsible for the antigen presentation of gluten peptides to the gluten-sensitive T cells in the small intestinal mucosa. This raises the possibility that the gluten antigens are presented in a different and more immunostimulatory way to the gluten-sensitive T cells in the small intestinal mucosa and perhaps HLA-DQ8 homozygosity is a marker of disease severity. However, there is no data to support this and a recent study suggests that HLA-DQ2 homozygosity leads to a higher prevalence of enteropathy associated T cell lymphoma from type 2 RCD [17]. Further work needs to be done to investigate the pathogenesis and the treatment of type 2 refractory coeliac disease.
Larger randomised controlled trials need to be done in the treatment of type 2 refractory coeliac disease. This may need the collaboration of several specialised centres in different countries to maximise the numbers available for treatment and follow up. In conclusion, our regime of the treatment of type 2 refractory coeliac disease with steroids and azathioprine appears safe and provides a significantly better prognosis than other proposed treatment algorithms.
References
1. Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. Lancet 2000; 356: 203-208.
2. Al-Toma A, Verbeek WH, Hadathi M, et al. Survival in refractory celiac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of singlecentre experience. Gut 2007; 56: 1373-1378.
3. Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterol 2009; 136: 81-90.
4. Maurino E, Niveloni S, Chernavsky AC, et al. Clinical characteristics and longterm outcome of patients with refractory sprue diagnosed at a single institution. Acta Gastroenterol Latinam 2006; 36:10-22.
5. Rubio-Tapia A, Kelly DG, Lahr BD, et al. Clinical staging and survival in refractory celiac disease: a single centre experience. Gastroenterol 2009; 136: 99- 107.
6. Gale J, Simmonds PD, Mead GM, et al. Enteropathy-type intestinal T-cell lymphoma:clinical features and treatment of 31 patients in a single center. J Clin Oncol 2000; 18: 795-803.
7. Daum S, Cellier C, Mulder CJJ. Refractory celiac disease. Best Pract Res Clin Gastroenterol 2005; 19: 413-424.
8. Al-Toma A, Verbeek WH, Mulder CJ. The management of complicated celiac disease. Dig Dis 2007; 25: 230-236.
9. Cellier C, Cerf-Bensussan N. Treatment of clonal refractory celiac disease or cryptic intraepithelial lymphoma: A long road from bench to bedside. Clin
Gastroenterol Hepatol 2006; 4: 1320-1321.
10. Goerres MS, Meijer JWR, Wahab PJ, et al. Azathioprine and prednisolone combination therapy in refractory celiac disease. Aliment Pharmacol Ther 2003; 18: 487-494.
11. Olaussen RW, Løvik A, Tollefsen S, et al. Effect of elemental diet on mucosal immunopathology and clinical symptoms in type 1 refractory celiac disease. Clin Gastroenterol Hepatol 2005; 3: 875-885.
12. Tuner SM, Moorghen M, Probert CS. Refractory coeliac disease: remission with infliximab and immunomodulators. Eur J Gastroenterol Hepatol 2005; 17: 667- 669.
13. Wahab PJ, Crusius JBA, Meijer JWR, et al. Cyclosporin in the treatment of adults with refractory celiac disease- an open pilot study. Aliment Pharmacol Ther 2000; 14: 767-774.
14. Al-Toma A, Goerres MS, Meijer JW, et al. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin Gastroenterol Hepatol 2006; 4: 1322-1327.
15. Al-Toma A, Visser OJ, van Roessel HM, et al. Autologous hematopoietic stem cell transplantation in refractory coeliac disease with aberrant T cells. Blood 2007; 109: 634-641.
16. Tack GJ, Wondergem MJ, Al-Toma A, et al. Autologous stem cell transplantation in refractory celiac disease type II patients unresponsive to cladribine therapy. DDW 2010 abstracts.
17. Al-Toma A, Goerres MS, Meijer JW, et al. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathyassociated T-cell lymphoma. Clin Gastroenterol Hepatol 2006; 4: 315-319.


Exploring cellular immune mechanisms in coeliac disease – innate cells, tTG-specific T cells and the effect of oats
Margaret Dunne1, Ross Comerford1, Louise Elliott2, Sarah Cooper2, Jacinta Kelly1, Conleth Feighery2
1 National Children’s Research Centre, Our Lady’s Children’s Hospital, Dublin, Ireland
2 Department of Immunology, St. James’ Hospital, Dublin, Ireland
Introduction
Our group has previously reported extensively on molecular mechanisms involved in coeliac disease (CD) pathogenesis – autoantibody recognition of tissue transglutaminase (tTG) [1,2], molecular markers of refractory CD [3] and changes in CD gut epithelial cell molecular signatures [4]. Current work is focussed on cellular mechanisms involved in CD – the involvement of innate cells, the investigation of T cells specific for tTG, and the effect of oats on immune cells.
Innate cells in CD
It has long been recognised that individuals with coeliac disease show perturbations in various immune cell populations, both in the periphery and intestine, suggesting involvement of such cells in disease pathogenesis. However, little is known regarding the specific role of innate cells such as dendritic cells and monocytes, or the so called innate lymphocytes; gamma delta T cells, mucosal associated invariant T (MAIT) cells, natural killer (NK) cells and invariant NKT cells. We aimed to define the frequency and phenotype of these innate lymphocytes in coeliac blood and gut, and identify populations that differ from healthy donors. Multicolour flow cytometry and immunofluorescent techniques were used to measure the frequency of innate lymphocyte subgroups in coeliac blood and intestinal tissue.
Preliminary work has uncovered significant differences in the frequency of certain innate cell populations between coeliac and healthy donors. Specifically, absolute numbers and frequency of CD11c+ cells, MAIT and gamma delta T cells were significantly decreased in the circulation of coeliac patients (Fig. 1 and 2).
While flow cytometric analysis shows a significant decrease in CD11c+ myeloid dendritic cells in the periphery, confocal analysis of CD11c+ cells in the gut shows a significant increase in cells in coeliac patients, particularly untreated patients, suggesting that these cells migrate to the gut from the periphery in active disease.

No significant changes were noted for plasmacytoid dendritic cells (CD123+), iNKT cells or NK cells (data not shown). In the case of gamma delta T cells, the observed reduction extended across all subsets (V delta 1, 2 and 3) and was even noted in patients adhering to a gluten-free diet. These early findings suggest involvement of such cells in coeliac disease. Current work is exploring whether cells decreased in the periphery are homing to the gut lesion, cell reactivity to gliadin, expression of tissue transglutaminase (tTG) and crosstalk with gliadin-reactive cells, to assess whether these cells play a direct role in gluten-mediated immune activation. Elucidation of such mechanisms may allow manipulation of innate lymphocytes for immunotherapy.
tTG-specific T cells
Another study is investigating the phenomenon of tTG-specific T cells. tTG is a ubiquitous multifunctional enzyme that plays a role in deamidating gliadin, thus facilitating its recognition by the immune system. Since a humoral response to tTG is evident (anti-tTG antibodies are a reliable, specific marker of CD), we hypothesised that a tTG-specific T cell response may also be mounted.
Peripheral blood cells cultured with tTG demonstrated a proliferative response in both coeliac and some healthy donors (Fig. 3).
The majority of cell lines grown from tTG-stimulated cells were CD4+ and produced pro-inflammatory IFN-, IL-17 and IL-21 upon stimulation. This preliminary work provides evidence for a tTG-specific T cell response with a pro-inflammatory phenotype, which may drive CD pathogenesis.


Toxicity of oats
Our group has an ongoing interest in the toxicity of prolamins other than gliadin in CD. Work is currently centred on evaluating changes in cells from duodenal biopsy before and after ingestion of oats. To date no significant differences have been noted using IN Cell analysis of cytoskeletal protein expression and morphological changes (Fig. 4). This work reinforces our earlier findings that oats may be acceptable in the coeliac diet [5].

Together, these studies aim to elucidate the cellular mechanisms involved in CD pathogenesis, with a view towards identifying novel biomarkers of disease or development of immunotherapeutic intervention strategies.
References
1. Byrne G, Feighery C, Jackson J, et al. Coeliac disease auto antibodies mediate significant inhibition of tissue transglutaminase. Clin Immunol 2010; 136: 426- 431.
2. Byrne G, Ryan F, Jackson J, et al. Mutagenesis of the catalytic triad of tissue transglutaminase abrogates coeliac disease serum IgA autoantibody binding. Gut 2007; 56: 336-341.
3. O'Shea U, Abuzakouk M, O'Morain C, et al. Investigation of molecular markers in the diagnosis of refractory coeliac disease in a large patient cohort. J Clin Pathol 2008; 61: 1200-1202.
4. Bracken S, Byrne G, Kelly J, et al. Altered gene expression in highly purified enterocytes from patients with active coeliac disease. BMC Genomics 2008; 9: 377.
5. Srinivasan U, Jones E, Carolan J, et al. Immunohistochemical analysis of coeliac mucosa following ingestion of oats. Clin Exp Immunol 2006; 144: 197-203.
When do antibody assays definitely predict coeliac disease?
Thomas Mothes
Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University Hospital Leipzig, Germany
Introduction
Results of antibody assays are becoming increasingly reliable in the diagnosis of coeliac disease (CD). In an interview, paediatric gastroenterologists requested a revision of the current diagnostic criteria of CD. Many of them want to omit the small bowel biopsy in symptomatic children with positive IgA antibodies against tissue transglutaminase (IgA-anti-tTG) or against endomysium (IgA-EmA), especially if they are DQ2/DQ8 positive [1]. The association of positive HLA DQ2/8 and serologic testing showed a high predictive value for CD and it was suggested that symptomatic children with high titres of IgA-anti-tTG could be diagnosed as CD patients without jejunal biopsy [2]. Alternatively, paediatric patients with high IgA-anti-tTGA level, in whom symptoms improve during a gluten-free diet (GFD), were suggested not to need a small intestinal biopsy to confirm CD [3]. According to new guidelines [4], in case of children and adolescents with signs or symptoms suggestive of CD and very high concentration of IgA-anti-tTG or antibodies against deamidated gliadin (exceeding 10 times upper limit of normal), the paediatric gastroenterologist may discuss with the parents and patients the option of performing further laboratory tests (EmA, HLA) in order to make the diagnosis of CD without biopsies. If so, antibody positivity should be verified by EmA-assay from a blood sample drawn at a separate occasion. If EmA testing confirms specific CD antibody positivity in this second blood sample, the diagnosis of CD can be made and the child started on a GFD. The new guidelines advise to check for HLA types in patients diagnosed without biopsy to reinforce the diagnosis of CD. The height of the stringent (ten times upper limit of normal) cut off was based on two publications applying one and the same test kit [5,6].
Patients and methods
We re-evaluated data of paediatric patients from recent publications [7-9] together with new data of patients from the Children’s Hospital of the Clinical Centre „St. Georg“ and the University children’s Hospital (Leipzig, Germany). Altogether sera of 256 coeliac and 603 control patients were included. Among the CD patients there were 2 children with latency, in whom the diagnosis was made later. Furthermore, 19 children with secretory IgA-deficiency (sIgAD) were among the CD patients. The high percentage of sIgAD (7.58 %) resulted from the inclusion of patients of a previous study on antibody assays in IgA-deficient children [8]. Since the normal frequency of sIgAD in CD is reported to be only 2.5 % [10], each CD patient with sIgAD was weighted only 1/3. All sera were withdrawn at the time of duodenal biopsy. All patients were biopsied under normal (gluten containing) diet due to suspicion of CD or
other gastrointestinal disorders. Intestinal pathology of all CD patients was in accordance with Marsh 2 or 3 criteria except the 2 patients with latency.
IgG antibodies against deamidated gliadin (analogous fusion) peptides (anti-GAF) and IgA anti-tTG were measured blinded in sera with tests from EUROIMMUN Medizinische Labordiagnostika (Lübeck, Germany).
Post-test probabilities were calculated for different pre-test probabilities and increasing cut-offs. Patients were considered as positive if either (A) IgA-anti-tTG or (B) IgGanti- GAF or (C) IgA-anti-tTG and IgG-anti-GAF or (D) IgA-anti-tTG and / or IgGanti- GAF were above the proposed cut-off. Alternatively (E), results of the two tests can also be variably combined. In this case the cut-off was defined as a straight line with negative slope in a diagram correlating the concentrations of IgA-anti-tTG and of IgG-anti-GAF. The result was regarded as positive if the following condition was fulfilled: units IgA-anti-tTG + 2 x units IgG-anti-GAF > cut-off.
Results
With increasing cut-off the specificity of all assays approaches 1. However, the sensitivity of the assays decreases strongly. Post-test probabilities strongly depend on pre-test probabilities. At a pre-test probability of 0.05, the assays of IgA-anti-tTG and IgG-anti-GAF exceed a post-test probability of 0.9 at 7 times the manufacturer’s cutoff.
This is true if the results of the single assays are evaluated (Fig. 1A, B) or if both tests are evaluated in combination (Fig. 1C, D). However, only if IgG-anti-GAF are included into the evaluation (either as single assay or in combination with IgA-antitTG), a post-test probability of 1 can be reached at higher cut-offs (above 9 times the manufacturer’s cut-off) (Fig. 1B, C).
The results can also be evaluated by a variable combination of the two tests according to procedure E (Fig. 1E). In this case a post-test probability of 0.9 is reached (at a pretest probability of 0.05) if both tests are above 5 times the manufacturer’s cut-off.
Alternatively, such a high post-test probability is also obtained if IgG-anti-GAF is above 7 times the manufacturer’s cut off at low IgA-anti-tTG or if IgA-anti-tTG is above 16 times the manufacturer’s cut-off at low level of IgG-anti-GAF.
Conclusions
The present data are calculated retrospectively and thus should be considered with caution. Further, post-test probabilities of 0.9 may not be sufficiently high to rely exclusively on antibody test results and to omit biopsies. However, the higher the posttest probabilities, the lower the fraction of coeliac patients, which can be diagnosed solely by means of antibody assays. Cut-offs allowing a definite diagnosis of coeliac disease and diagnostic strategies of combination of several assays have to be evaluated more carefully in systematic prospective studies with a large number of patients for the different assays on the market.

Acknowledgements
This analysis represents the preparatory work for a larger study, which will be performed in cooperation with the Working Group on CD of the German speaking Society for Paediatric Gastroenterology and Nutrition (GPGE). T. Richter (Children’s Hospital of the Clinical Centre „Sankt Georg“ Leipzig, Germany) and H. H. Uhlig (Translational Gastroenterology Unit, Experimental Medicine, University of Oxford, John Radcliffe Hospital Oxford, U.K. and University Children‘s Hospital Leipzig, Germany) is thanked for patients’ sera and diagnoses not included in the above mentioned previous publications. C. Dähnrich (EUROIMMUN Medizinische Labordiagnostika GmbH Lübeck, Germany) is thanked for antibody assays. The support by EUROIMMUN Medizinische Labordiagnostika (Lübeck, Germany) is gratefully acknowledged.
References
1. Ribes-Koninckx C, Mearin ML, Korponay-Szabó I, et al. Coeliac disease diagnosis: ESPGHAN 1990 Criteria or need for a change? Results of a questionnaire. J Pediatr Gastroenterol Nutr 2012; 54: 15-19.
2. Clouzeau-Girard H, Rebouissoux L, Taupin J-L, et al. HLA-DQ Genotyping combined with serological markers for the diagnosis of celiac disease: Is Intestinal Biopsy Still Mandatory? J Pediatr Gastroenterol Nutr 2011; 52: 729-733.
3. Mubarak A, Wolters VM, Gerritsen SAM, et al. A Biopsy Is Not Always Necessary to Diagnose Celiac Disease. J Pediatr Gastroenterol Nutr 2011; 52: 554-557.
4. Husby S, Koletzko S, Korponay-Szabo IR, et al. ESPGHAN guidelines for the diagnosis of coeliac disease in children and adolescents. An evidence-based approach. J Pediatr Gastroenterol Nutr 2012; 54: 136-160.
5. Hill PG, Holmes GKT. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther 2008; 27: 572-577.
6. Dahlbom I, Korponay-Szabó IR, Kovács JB, et al. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. Pediatr Gastroenterol Nutr 2010; 50: 140-146.
7. Prause C, Richter T, Koletzko S, et al. New developments in serodiagnosis of childhood celiac disease: assay of antibodies against deamidated gliadin. Annals N Y Acad Sci 2009; 1173: 28-35.
8. Villalta D, Tonutti E, Prause C, et al. IgG-antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA-deficiency. Clin Chem 2010; 56: 464-468.
9. Richter T, Bossuyt X, Vermeersch P, et al. Determination of IgG and IgA antibodies against native gliadin is not helpful for the diagnosis of coeliac disease in children up to 2 years of age. J Pediatr Gastroenterol Nutr (in press).
10. Green PHR, Cellier C. Celiac Disease. New England J Med 2007; 357: 1731- 1743
New antibodies against human tissue transglutaminase as research tools
Johannes Wolf, Thomas Mothes
Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics,
University Hospital Leipzig, Germany
Introduction
Tissue transglutaminase (tTG) as member of the transglutaminase (TG) family is a key player in the pathogenesis of coeliac disease (CD) [1]. CD is associated with other disorders like dermatitis herpetiformis (DH) and neuronal diseases [2,3]. Additionally tTG is supposed to be involved in further disease processes, e.g. cancer or neurodegenerative disorders like Alzheimer`s and Parkinson`s disease [4,5]. For investigation of the role of tTG, antibodies are necessary, which are highly specific in terms of recognition of tTG but not of other TGs. Furthermore, test systems applying these antibodies should exhibit a high analytical sensitivity. Whereas a large number of different polyclonal antibodies are available, the choice of monoclonal antibodies (mabs) is limited. Most antibodies hitherto available were raised against the guinea pig immunogen. Our aim was to characterise a panel of new mabs raised in mice against human recombinant tTG (rhutTG), to study their application in ELISA, immunoblot and immunohistochemistry and to compare them with two widely used commercially available mabs (CUB7402 and TG100) generated by immunisation with guinea pig tTG. Due to amino acid sequence similarities, these mabs are known to react with the human tTG but may also bind unspecifically to other members of the TG family [6].
Materials and methods
Generation of mabs to human tTG was published in a previous report [7]. Isotype was assessed applying a monoclonal antibody isotyping kit from Pierce (Bonn, Germany).
Affinity of the mabs was determined by an indirect ELISA with microtitre plates coated overnight (ON) at 4 °C with 2 μg/mL of rhutTG in carbonate buffer and blocked with 2 % Tween in phosphate buffered saline (PBS) at room temperature (RT). Purified mabs were diluted (500 to 0.425 ng/mL) and incubated in Tris-buffered saline supplemented with 0.05 % Tween (TBST). Subsequently, horse radish peroxidase (HRP)-conjugated goat anti-mouse IgG (H+L) from Dianova (Hamburg, Germany) (1:2000) was added and incubated at RT. Finally, HRP-reaction was visualized using a TMB-staining kit (ajRoboscreen, Leipzig). The reaction was stopped with 150 μL of 0.5 mol/L H2SO4 and the absorbance was determined at 450 nm.
RhutTG and tissue homogenates of human neocortex samples (acquisition of patients personal data, autopsy, and handling of autopsy material approved by the ethical committee of the University of Leipzig, no. 063/2000 ) were separated by non-reducing electrophoresis in 10 % pre-cast Tris-glycine polyacrylamide gels (Serva, Heidelberg) and transferred onto nitrocellulose membranes. Membranes were blocked with TBST containing 5 % skim milk (blocking buffer), incubated ON at RT with different mabs and subsequently with HRP-conjugated goat anti-mouse IgG (1:2000). Bound HRP was visualised with chemoluminescence substrate [6].
Epitopes were defined by pepscans applying decapeptides (spotted to nitrocellulose membranes, spanning the complete sequence of tTG, overlapping by two amino acids) (Core Unit Peptide Technologies of the IZKF Leipzig, Germany). After washing in methanol und TBST, the membranes were blocked with blocking buffer, washed and incubated subsequently with the different mabs (3 μg/mL in blocking buffer) ON at RT. After further washing, membranes were incubated with secondary antibody as described above. After final washing, luminescence was detected using the chemoluminescence substrate solution. The binding of mabs to the peptides was evaluated visually.
Binding of antibodies to tissue sections from monkey intestine (EUROIMMUN, Lübeck) was investigated by indirect immunofluorescence microscopy. Anti-tTG mabs, monoclonal mouse anti-beta actin antibody (Sigma-Aldrich, Taufkirchen, Germany) and an isotype control (15 μg/mL in PBS supplemented with 0.2 % Tween) were incubated according to the instructions of the manufacturer. After washing, the tissue sections were incubated with Alexa 488-conjugated goat anti-mouse IgG (1:200, Invitrogene, Darmstadt, Germany). After final washing in PBS supplemented with 0.2 % Tween and Evans blue, the slides were covered with glycerol/gelatine (Sigma- Aldrich) containing 4',6-diamidino-2-phenylindole and triethylenediamine (Serva, Heidelberg). For microscopy an Axioskop microscope (Carl Zeiss, Jena, Germany) was used. For control, serum of a CD patient under normal (gluten-containing) diet with high concentration of IgA anti-tTG was applied (1:100 in PBS with 0.2 % Tween).
Results and discussion
From our mabs, 10F3, 5F3, and 3C4 showed the highest affinity towards rhutTG, coated to the microplate surface. The affinities of TG100 and CUB7402 were comparable (Fig. 1A). After blotting of electrophoretically separated rhutTG to nitrocellulose, all mabs stained a band corresponding to the molecular weight of hutTG (Fig. 1B). The intensity of staining did not correlate with ELISA results (Fig. 1A). Although 5 or even 10fold higher amounts of tTG were applied, staining was weaker with 5F3 or 3C4, respectively, than with 10F3, TG100 and CUB7402. These findings indicate different binding to native and SDS treated tTG. All mabs displayed a clear reactivity with tTG from human brain except 3G9 and 3C4, which stained a very weak or no tTG band, respectively, despite high protein concentrations (Fig. 1C).
CUB7402 stained a second band with a molecular weight below 50 x 103. This observation could be reproduced in four further cerebral cortex samples (not shown).
Dual incubation of the membrane with mab 3C10 and a monoclonal anti-beta actin antibody revealed a staining, which could be completely merged with that of CUB7402 (Fig. 1D) suggesting unspecific binding of anti-guinea pig tTG antibody to cellular actin. On closer inspection, very faint bands in this region could also be realised with mabs 3G9 and 8F11.

The high background staining produced by 3G9 and 8F11 may be due to the fact that these two mabs are isotype IgG2 whereas all other mabs were defined as Isotype IgG1 (Tab. 1). Epitope mapping of these two illustrated more than fifty randomly distributed spots on the pepscan membrane with highest reactivity in domain 2 of hutTG, the catalytic core.

Pepscan analysis of all other mabs revealed distinct sequences 6 to 12 amino acids long. 10F3 and 3C4 exhibited two or three epitope sequences, respectively, whereas the other antibodies recognised only one (Tab. 1). The epitopes of most (7 of 9) mabs were located in the domain 2 of tTG (Fig. 2). In accordance with our observation, it was found that IgA anti-tTG response in patients with CD is focused on the catalytic core of the antigen [8,9], causing inhibition of enzyme activity [9]. The reactivity of single-chain antibody fragments against tTG derived from phage libraries was also found to be restricted to the enzymatic core region [8].

The finding that 3C4 recognises three sequences far apart from each other argues for a conformation epitope and might be the reason for the weak reactivity in Western blotting. The epitope of CUB7402 was shorter at the C-terminal end (by 22 amino acids) than mentioned by the manufacturer. This may be due to different length of amino acid sequences applied for epitope testing. For final confirmation of the epitopes, inhibition experiments are necessary using synthetic peptides with the respective amino acid sequence.
To further characterise the specificity of the mabs, binding to tissue sections of monkey intestine was investigated. A typical staining pattern was obtained applying a CD patient’s serum containing IgA anti-tTG antibodies (Fig. 3A). Connective tissue of villi and crypts (Lamina propria mucosae) as well as submucosal vessels were stained.
A very similar fluorescence pattern was observed for 10F3 (Fig. 3B) and TG100 (not shown).
However, structures within the epithelium were also stained very weakly. This epithelial staining was much more pronounced for CUB7402 (Fig. 3C). The epithelial staining obtained with the mabs raised against tTG was compared with that produced a mouse antibody against beta actin. The fluorescence pattern elicited by the anti-beta actin antibody was comparable with that produced by CUB7402 (Fig. 3D). This might raise the question of cross-reactivity of antibodies to tTG with cellular actin.
Previously, reorganisation of actin in cytoskeleton of endothelial and colonic epithelial cells after treatment with CUB7402 und CD sera was described [10,11]. It remains to be examined if this could (at least partially) be due to binding of antibodies to actin.

To conclude, the new mabs 10F3 and 3C10 exhibited a high affinity to native and SDS-treated tTG without evidence of cross-reactivity with other cellular proteins and are comparable with TG100. The new mabs represent useful tools for investigation of pathoprocesses not only in CD, but also in other disorders like neurological diseases.
Acknowledgements
Thanks to Awad A. Osman and Ingolf Lachmann (AJ Roboscreen GmbH Leipzig, Germany) for monoclonal antibodies raised against tTG and to EUROIMMUN Medizinische Labordiagnostika for tissue sections. Thomas Arendt (Paul Flechsig Institute for Brain Research, University of Leipzig, Germany) is thanked for providing the human brain samples. This work was supported by the Free State of Saxony and European Union (European Fund for Regional Development / EFRE).
References
1. Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997; 3: 797-801.
2. Marietta MV, Camilleri MJ, Castro LA, et al. Transglutaminase autoantibodies in dermatitis herpatiformis and celiac sprue. J Invest Dermatol 2008; 128: 332-335.
3. Kim SY, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem Int 2002; 40: 85-103.
4. Mehta K, Kumar A, Kim HI. Transglutaminase 2: A multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol 2010; 80: 1921-1929.
5. Jeitner TM, Pinto JT, Krasnikov BF, et al. Transglutaminase activation in neurodegenerative diseases. J Neurochem 2009; 109: 160-166.
6. Wolf J, Lachmann I, Wagner U, et al. Quantification of human tissue transglutaminase by luminescence sandwich enzyme-linked immunosorbent assay.
Anal Biochem 2011; 419: 153-160.
7. Wolf J, Lachmann I, Wagner U, et al. Immunoassay of in vitro activated human tissue transglutaminase. Anal Biochem 2011; 411: 10-15.
8. Sblattero D, Florian F, Azzoni E, et al. The analysis of the fine specificizy of celiac disease antibodies 16using tissue transglutaminase fragments. Eur J Biochem 2002; 269: 5175-5181 .
9. Byrne G, Ryan F, Jackson J, et al. Mutagenesis of catalytic triad of tissue transglutaminase abrogates coelic disease serum IgA autoantibody binding. Gut 2007; 56: 336-341.
10. Myrsky E, Kaukinen K, Syrjänen M, et al. Coelic disease-specific autoantibodies targeted against transglutaminase 2 disturb angiogenesis. Clin Exp Immunol 2008; 152: 111-119.
11. Ferrara F, Quaglia S, Caputo I, et al. Anti-transglutaminase antibodies in noncoeliac children suffering from infectious diseases. Clin Exp Immunol 2010; 159: 217-223.
Risk assessment of gluten exposure among coeliac patients
Astrid G. Kruizinga1, Anna Gibert2, Carlo Catassi3
1 TNO, Zeist, the Netherlands
2 Associació Celíacs de Catalunya, Barcelona, Spain
3 Department of Pediatrics, Università Politecnica delle Marche, Ancona, Italy and Center for Celiac Research, University of Maryland School of Medicine, Baltimore MD (USA)
Introduction
The regulatory environment in relation to the composition and labelling of foodstuffs suitable for people intolerant to gluten has changed recently. In 2008, the Codex Committee on Nutrition and Foods for Special Dietary Uses set new definitions and stricter limits on the amount of gluten allowed in foods. In 2009 this was also incorporated in EU regulation (EC/41/2009). After two years of products in the market following the new regulation, we investigated the following question: What is the percentage of the coeliac disease (CD) population at risk for an adverse effect due to the consumption of gluten-free and very low gluten dietetic foods in a specific country?
Methodology
TNO has developed an approach for allergen risk assessment using probabilistic techniques in order to accurately define potential health problems upon exposure to allergens [1-4]. Probabilistic modeling is now considered to be the most promising approach for use in population risk assessment [5]. It has been shown that this method is also applicable to gluten [6].
Fig. 1 shows the schematised presentation of the probabilistic approach in food allergy risk assessment. In this approach several input variables are included: consumption of the food at an individual meal/eating occasion (i.e. the chance that an allergic person consumes a certain product and in which amount this product is consumed) and the concentration of the allergen in the food (i.e. whether this food contains the allergen and in which concentration). These input variables determine the exposure to the allergen. Also, the threshold distribution is determined. By comparing the distribution of the thresholds to the distribution of the exposure to the allergen, the probability of an allergic reaction is determined.

Threshold data
In 2007, the effects of long-term low-dose gluten challenge have been demonstrated [7]. In this trial 49 adults with biopsy-proven CD who adhered to a gluten-free diet for over two years were challenged. The majority of the subjects in the 50 mg/day group showed a worsening in morphometric parameters. This dose is therefore the LOAEL, meaning that the threshold would be 50 mg/day or lower. As no statistical differences in morphometric parameters have been observed in the 10 mg/day group, this dose is considered to be a NOAEL. Based on this study, a threshold between >10 and ≤50 mg/day might be assumed [7]. However, no information about the distribution of individual thresholds in the CD population is available. Also, no information was available with respect to whether the population threshold is either 10 mg gluten per day, 50 mg gluten per day or a dose between 10 and 50 mg per day. Therefore, the risk assessment was performed for the NOAEL (10 mg/day) and the LOAEL (50 mg/day) and some dose in between and a normal distribution with a mean of 30 mg and a standard deviation of 10 mg.
Exposure to gluten
The exposure to gluten was estimated by using data on consumption of gluten-free foods that are available from a food consumption survey performed in four European countries (Italy, Spain, Norway and Germay) among people on a gluten-free diet [8]
combined with data on concentrations of gluten in gluten-free products in these countries specifically collected for this research.
Results and conclusion
For each country (Spain, Italy, Norway and Germany) the data regarding the food consumption and concentration from one specific country were combined with the threshold scenarios (distribution, 10 mg, 20 mg, 30 mg, 40 mg and 50 mg). The results are shown in Fig. 2. Overall, the percentage of the CD population at risk is below the 0.1 %, with the exception of Italy, where the percentage at risk is 0.16 %. When assuming a point estimate for the sensitivity at a threshold of 10 mg gluten per day, the risk is still below the 0.2 % for each of the countries investigated. With the exception of Italy, the higher thresholds (i.e. 20, 30, 40 and 50 mg/day) did not result in a quantifiable risk for the specific countries. In Italy the risk at 10 mg/day was higher (around 0.5 %), probably due to the high consumption of gluten-free foods, compared to the other countries.

These data show that the risk of an adverse reaction in coeliac patients due the consumption of gluten free foods in the current market is very small.
References
1. Houben G, Penninks A, Brussaard T. Changes in practice of risk assessment: from possibility to probability. In: TNO International Food Allergy Forum April 2002, the Netherlands, 15-16.
2. Spanjersberg MQI, Kruizinga AG, Rennen MAJ et al. Risk assessment and food allergy: the probabilistic model applied to allergens. Food Chem Toxicol 2007; 45: 49-54.
3. Kruizinga AG, Briggs D, Crevel RWR et al. Probabilistic risk assessment model for allergens in food: sensitivity analysis of the minimum eliciting dose and food consumption. Food Chem Toxicol 2008; 46: 1437-1443.
4. Spanjersberg MQI, Kruizinga AG, Knulst AC et al. Quantitative risk assessment reveals the consequences of undeclared allergens in food products: a case study. Submitted to Food Chem Toxicol.
5. Madsen CB, Hattersley S, Buck J et al. Approaches to risk assessment in allergy: Report from a workshop ‘Developing a framework for assessing the risk from allergenic foods. Food Chem Toxicol 2009; 47: 480-489.
6. Kruizinga AG, Spanjersberg MQI, Houben GF. Risk Assessment principles for allergens in food: application to gluten. In: Proceedings of the 23rd Meeting of the Working Group on Prolamin Analysis and Toxicity. Spain. 25-27 September 2008, pp. 115-121.
7. Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebocontrolled trial to establish a safe gluten threshold for patients with coeliac
disease. Am J Clin Nutr 2007; 85: 160-166.
8. Gibert A, Espadaler M, Canela MA et al. Consumption of gluten-free products: should the treshold value for trace amounts of gluten be at 20, 100 or 200 ppm? Eur J Gastroenterol Hepatol 2006; 18: 1187-1195.
Cereal triggers of innate immune activation
Detlef Schuppan1,2, Yvonne Junker, Victor Zevallos1,2, Herbert Wieser3
1 Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, USA
2 Division of Molecular and Translational Medicine, Dept. of Medicine I, Johannes Gutenberg University, Mainz, Germany
3 German Research Centre of Food Chemistry and Hans-Dieter-Belitz-Institute for Cereal Grain Research, Freising, Germany
Introduction
Coeliac disease (CD) is defined as a small intestinal inflammatory disease caused by an adaptive, Th1 T cell mediated immune response against ingested gluten (storage proteins) in wheat, barley and rye. Coeliac patients carry HLA-DQ2 or -DQ8 as major genetic predisposition and develop a characteristic mucosal (IgA) autoantibody response to the enzyme tissue transglutaminase which potentiates immunogenicity of certain gluten epitopes via deamidation of glutamine residues [1].
The well-defined role of adaptive immunity in CD contrasts with that of innate immunity to the ingested cereals which has been shown to exist but whose molecular players remain largely elusive. Thus, peptide p31-43 (or p31-49) from -gliadin that lacks adaptive stimulatory capacity was described as trigger of innate immunity as it induced IL-15 and Cox-2 expression in biopsy cultures from CD patients [2] and MHC class I polypeptide-related sequence A (MICA) on intestinal epithelial cells [3]. But these results have been difficult to reproduce in cell culture and no receptor for these effects could be identified. Moreover, gliadin and peptides different from p31-43 were reported to induce expression of costimulatory molecules and proinflammatory cytokines in monocytes and dendritic cells [4-7], and the chemokine receptor CXCR3 was implicated in increased intestinal epithelial permeability upon gliadin challenge in a MyD88-dependent manner [8-9]. In all cases, the responsible gliadin peptide was not reproducibly identified, and CXCR3 as signaling receptor lacked plausibility. Taken together, a clear picture of the role of the innate immune system in coeliac disease had not emerged.
Innate immunity serves as early response system to mainly microbial and chemical stimuli. It also contributes a successful priming of adaptive immunity [10]. Major cells of innate immunity are macrophages, monocytes, dendritic cells and polymorphonuclear leukocytes that upon pathogen contact release proinflammatory cytokines and chemokines via their pattern recognition receptors, such as toll-like receptors (TLRs) [11], amplifying the immune response via recruitment and activation of additional inflammatory cells.
Our preliminary studies showed that neither pepsin-trypsin (PT) digested gliadin nor p31-43 elicited innate immune responses in several colonic or intestinal epithelial cell lines. However, PT gliadin (but not p31-43) strongly activated proinflammatory cytokine release from mouse and human monocytes, macrophages and dendritic cells.
This activity did not reside in any gliadin fraction (α, γ, ) [12], but was found in a class of (contaminating) non-gluten proteins, namely the wheat amylase-trypsin inhibitors (ATIs). We could also demonstrate that ATIs noncovalently interact with omega gliadins and trigger these responses via toll like receptor 4 which has been known to transmit innate signals after binding to bacterial lipopolysaccharide but also to some other ligands.
Each wheat variant contains up to 11 known ATIs with molecular weights between 12 x 103 and 16 x 103 which share modest sequence similarity but a highly conserved secondary structure, based on five intrachain disulfide bonds and four α-helices, endowing them to form mono- to tetramers [13,14]. Rye and barley contain similar ATIs which the cereal plants employ to fend off parasites, such as the common mealbug, e.g., by inhibiting the parasites’ digestive enzymes. ATIs largely resist intestinal degradation and stimulate mucosal cytokine release after feeding in vivo.
Furthermore, they have been identified as major allergens in baker’s asthma which affects up to 8 % of bakers within two years after starting their profession [15].
Finally, ATI content in cereal protein appears to have increased dramatically with (resistance) breeding of grains. Notably, ATI content and activity in cereals appears to go hand in hand with gluten content.
Apart from likely serving as the major innate immune trigger in CD, ATIs may have a further reaching pathogenic role. An example is the emerging epidemic of non-coeliac gluten sensitivity which according to some estimates may reach 5 - 7 % in wheat consuming populations [16,17]. This condition, which is currently diagnosed by exclusion and re-occurence of symptoms after re-exposure to gluten, lacks overt duodenal histological changes. We have also observed this lack of histological damage after ATI challenge, despite an increased production of proinflammatory cytokines.
Similarly, in gluten sensitivity a “subliminal” inflammatory reaction to “gluten ingestion” has been suspected [16-18]. Further in vitro, animal experimental and human studies are on the way to further explore the suspected role of cereal ATIs in CD, in gluten sensitivity and other intestinal inflammatory disorders of the GI tract, and in general autoimmunity.
References
1. Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 2009; 137: 1912-1933.
2. Maiuri L, Ciacci C, Ricciardelli I, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 2003; 362: 30-37.
3. Hue S, Mention JJ, Monteiro RC, Zhang S, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004; 21: 367-377.
4. Cinova J, Palova-Jelinkova L, Smythies LE, et al. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol 2007; 27: 201-209.
5. Jelinkova L, Tuckova L., Cinova J, et al. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett 2004; 571: 81-85.
6. Nikulina M, Habich C, Flohe SB, et al. Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol 2004; 173: 1925-1933.
7. Tuckova L, Novotna J, Novak P, et al. Activation of macrophages by gliadin fragments: isolation and characterization of active peptide. J Leukoc Biol 2002; 71: 625-631.
8. Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterol 2008; 135: 194-204.
9. Thomas KE, Sapone A, Fasano A, et al. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are
MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol 2006; 176: 2512-2521 .
10. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20: 197-216.
11. Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol 2005; 17: 1-14.
12. Wieser H, Koehler P. The biochemical basis for celiac disease. Cereal Chem 2008; 85: 1-13.
13. Tatham AS, Shewry PR. Allergens to wheat and related cereals. Clin Exp Allergy 2008; 38: 1712-1726.
14. Oda Y, Matsunaga T, Fukuyama K, et al. Tertiary and quaternary structures of 0.19 alpha-amylase inhibitor from wheat kernel determined by X-ray analysis at 2.06 A resolution. Biochemistry 1997; 36: 13503-13511.
15. Walusiak J, Hanke W, Górski P, et al. Respiratory allergy in apprentice bakers: do occupational allergies follow the allergic march? Allergy 2004; 59: 442-50.
16. Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the "no man's land" of gluten sensitivity. Am J Gastroenterol 2009; 104: 1587-1594.
17. Troncone R, Jabri B. Coeliac disease and gluten sensitivity. J Intern Med 2011; 269: 582-590.
18. Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placeboontrolled trial. Am J Gastroenterol 2011; 106: 508-514.
VI. Gluten in food technology
Gluten processing in food technology
Jussi Loponen
Fazer Group, Vantaa, Finland
Five meanings for gluten
Gluten is an ambiguous term as it has multiple meanings in food technology (Tab. 1). Firstly, in the terminology of cereal biochemistry, the main storage proteins of wheat kernel are called gluten proteins. In this case, the gluten proteins cover both the gliadins and the glutenins, and the classification is based on the protein structure. The glutenins are polymeric as they can form intermolecular disulphide bonds whereas the gliadins are monomeric as they contain only intramolecular disulphide bonds. Based
on the molecular weight of subunits, the glutenins can be further divided to highmolecular- weight (HMW) and low-molecular-weight (LMW) glutenin subunits [1].
Similarly gliadins are grouped to -, -, and -gliadins. In another classification system, the gluten proteins are classified into three prolamin groups: HMW prolamins, sulphur-rich prolamins, and sulphur-poor prolamins. Glutamine (>30 mol-%) and proline (>10 mol-%) are the most frequently occurring amino acids of gluten proteins.
HMW glutenin subunits, however, are also rich in glycine (~18 mol-%), which differentiates them from the primary structure of other wheat prolamins. On the other hand, the -gliadins contain no cysteine, which practically makes them the sulphurpoor prolamins of wheat.
The second meaning of gluten is from baking technology where the term gluten stands for the viscoelastic component of wheat dough. The viscoelastic feature of wheat dough is unique as no other cereal proteins can form such cohesive structures.
Continuous viscoelastic gluten network is formed when wheat flour is mixed with water. Gluten network structure is based on disulphide linkages and hydrogen bonding. Glutenins are mainly responsible for the elasticity and strength of the dough whereas gliadins are the viscous component of gluten network. In bread making, the gluten network is responsible for the water and gas holding in dough system and, thus, plays an important role in the dough volume increase during dough proofing as a consequence of CO2-production by baker’s yeast. During oven baking, gluten proteins denaturate, and the water retained in gluten is taken up by gelatinising starch. This exchange of water between gluten and starch is an extremely important phenomenon in wheat dough systems that seems to be fairly difficult to mimic, for instance, in gluten-free baking protocols. In gluten-free baking, gluten is replaced with water absorbing hydrocolloids that, however, do not donate the water to starch during oven baking in the same manner as gluten. This is the reason why gluten-free recipes often are high in free-water available for starch gelatinisation.
Third meaning of gluten is in the context of coeliac disease, where the term gluten refers to the prolamin proteins of specifically wheat, barley, rye and their relative cereal grains. In celiac disease, the gluten is the primary trigger of the disease. Fourth meaning of gluten is in the gluten-starch separation, where gluten refers to the coproduct of the process, vital wheat gluten. Vital wheat gluten may be further be processed to gluten ingredients with increased functionalities i.e. modified/soluble gluten (fifth meaning of gluten).

Influencing the strength of the gluten network in wheat dough
A straightforward way to either weaken or strengthen the gluten network is to affect its disulphide bonding. The formation of disulphide bonds can be promoted through oxidation reactions, for instance, by using glucose oxidase enzyme. This enzyme indirectly induces gluten oxidation as the oxidation of glucose produces hydrogen peroxide as the side product that can further induce oxidation of free thiol groups to disulphide bonds. On the other hand, reduction of disulphide bonds as well as enzymatic hydrolysis of peptide bonds obviously results in depolymerisation and degradation of the polymeric network that weakens the dough. In certain applications, such as cracker baking, controlled weakening of gluten may be a desired property.
Reducing agents, such as cysteine, or mild protein hydrolysis may be used to achieve a controlled weakening of gluten, which will increase the gas leakage from the dough structure and, thus, reduce volume increase during proofing. Certain yeast preparations or lactobacilli may be used to induce weakening of gluten network as they provide or generate active glutathione (GSH, a reducing agent) to the dough system.
Gluten depolymerisation and degradation in sourdoughs
One mode of hydrolysis and depolymerisation of wheat gluten proteins occurs during wheat sourdough fermentation [2]. This is illustrated in Fig. 1. During the first hours of fermentation the polymeric HMW glutenins undergo depolymerisation, which is seen as their shift from low-soluble fraction to alcohol-soluble protein fraction. As the fermentation proceeds and acidity increases close to pH 4, the HMW glutenins start to degrade and disappear with simultaneous formation of a new 30 x 103 MW protein band, which has been shown to be a hydrolysis fragment from HMW glutenins. In wheat sourdough, the disulphide reducing activity likely originates from endogenous GSH of wheat flour that is kept in active mode by sourdough lactobacilli with GSHreductase activity; the proteolytic activity, in turn, originates mostly from endogenous
aspartic endopeptidases of wheat flour [3].

The most convenient way to manage (either control or promote) the proteolysis in sourdoughs is to adjust the proteolytic activity of flours used. Whereas heat treatments of seeds, such as those used in flaking, lower the activity of endogenous enzymes, industrial seed germination (malting) increases the proteolytic activity of cereal seeds and flours obtained thereof. Thus, by using cereal malts as a raw material in sourdough fermentation, a more extensive proteolysis is achievable. This may be benefitted for instance to increase the flavour formation in sourdough baking as some of the amino acids released during the sourdough proteolysis act as precursors for typical bread flavour compounds [5]. On the other hand, an extensive hydrolysis of prolamins during sourdough fermentation may be used to reduce the amount of immunogenic prolamin structures. For instance, in rye malt sourdoughs 99.5 % of the rye prolamins were hydrolysed to an extent that they no more were detectable with the immunoassays utilising the R5 antibody [6]. Despite the extensive hydrolysis, the residual prolamin content of such pre-ferments is still >300 mg/kg, which means that as such its use in gluten-free food applications is not possible. What, however, is possible is to further eliminate the residual prolamins by physical treatments or added peptidase preparations. The obvious benefit of applying such malt ingredients in gluten-free applications would be the addition of whole grain components (dietary fibre components, micronutrients) to the gluten-free recipies as well as the generation of aroma. Aroma arises especially from the high levels of free amino acids in the system after extensive proteolysis. The amino acids serve as flavour precursors as indicated above.
Gluten as food ingredient – versatility requires modification
Gluten is a co-fraction derived from wheat starch manufacture process, and this kind of gluten is called vital wheat gluten. Vital wheat gluten is mainly used in bread recipes, where it is added to improve the dough handling and bread volume properties. Vital wheat gluten is a non-soluble and non-dispersible ingredient, and this greatly limits its use in most food technology applications. The production of vital wheat gluten is, however, massive in scale and obviously new fields of applications for its industrial utilisation are sought. One way to increase its industrial use is to develop gluten ingredients with improved functionalities. Increasing the solubility of gluten or its dispersion and surface properties would all be added functional values for gluten.
The most common way to increase the solubility and dispersion properties of gluten is by enzymatic hydrolysis. In addition to enzymatic treatments, also chemical treatments can be used to produce modified gluten. Chemical treatments can also be used to elicit hydrolysis or another kind of modification, deamidation. Deamidation of gluten practically means the conversion of its glutamine to glutamic acid residues. Glutamic acid carries a negative charge under non-acidic conditions (pKa 4.15) and, thus, increases the negative charge of gluten proteins. The increase in charge properties augments the interactions of gluten with water and also makes the gluten more soluble and also surface active as hydrophilic regions are introduced to the predominantly hydrophobic protein molecules. Increases in the surface activity practically mean, that the emulsion and foam properties are improved, which positively influences the versatile usage of gluten in different food applications. Deamidation of gluten can be achieved also enzymatically by protein glutaminase [7].
The modified gluten ingredients may be used in many food applications. This means that their use in other than cereal food processes is very likely. Soluble or surface active gluten ingredients may be added, for instance, to meat or confectionery products i.e. to product types that consumers can automatically consider being gluten-free.
Moreover, the deamidated gluten proteins and peptides are not detectable by using the immunochemical gluten detection assays (for more detailed information see [8]).
Limited detection of gluten, which likely is the case also in gluten hydrolysates, can turn out to be a safety risk considering the coeliac disease patients’ everyday life and food choices.
References
1. Shewry PR, Tatham AS, Forde J. et al. The classification and nomenclature of wheat gluten proteins: a reassessment. J of Cereal Sci 1986; 4: 97-106.
2. Loponen J, Salovaara H, Sontag-Strohm T, et al. Degradation of HMW glutenins during wheat sourdough fermentations. Cereal Chem 2004; 81:87-93.
3. Gänzle MG, Loponen J, Gobbetti M. Proteolysis in sourdough fermentations: mechanisms and potential for improved bread quality. Trends Food Sci Tech 2008; 19: 513-521.
4. Loponen J. Prolamin degradation in sourdoughs. EKT-sarja 2006; no. 1372, Helsingin Yliopisto, Helsinki.
5. Hansen Å, Schieberle P. Generation of aroma compounds during sourdough fermentation: applied and fundamental aspects. Trends Food Sci Tech 2005; 16: 85-94.
6. Loponen J, Kanerva P, Zhang C, et al. Prolamin hydrolysis and pentosan solubilization in germinated-rye sourdoughs determined by chromatographic and immunological methods. J Agric Food Chem 2009; 57: 746-753.
7. Yong YH, Yamaguchi S, Matsumura Y. Effects of enzymatic deamidation by protein-glutaminase on structure and functional properties of wheat gluten. J Agric Food Chem 2006; 54: 6034-6040 .
8. Kanerva P, Brinck O, Sontag-Strohm T, et al. Deamidation of gluten proteins and peptides decreases the antibody affinity in gluten analysis assays. J Cereal Sci 2011; 53: 335-339.
Advances in the production of gluten-free breads
Elke K. Arendt
School of Food and Nutritional Sciences, University College Cork, Ireland
Permanent lifelong withdrawal of gluten from the diet is the only effective treatment for coeliac disease (CD). However, removal of gluten from bread formulations often results in a liquid batter, rather than a dough system during the pre-baking phase, and can result in baked bread with crumbling texture, poor colour and other quality defects (Fig. 1). Indeed, gluten is the main structure forming protein present in wheat flour, and plays a major role in bread-making functionality of wheat flours by providing visco-elasticity to the dough, good gas holding properties, and good crumb structure of many baked products (Fig. 2). Currently many of the gluten-free (GF) baked products that are available on the market are of low quality, exhibiting poor mouth-feel and flavour. These problems present major technological challenges to both the cereal technologist and the baker, and have led to the search for alternatives to gluten in the manufacture of GF baked products. GF bread requires polymeric substances that mimic the viscoelastic properties of gluten in bread dough. The production of GF breads mainly involves the incorporation of starches, protein-based ingredients like dairy proteins and hydrocolloids into a GF base flour (Fig. 3) that could mimic the viscoelastic properties of gluten and result in improved texture, mouthfeel, acceptability and shelf-life of these products. It is recommended to use a range of GF flours rather than just one flour to achieve products of good sensorial and textural properties. The addition of a certain percentage of starch to a GF formulation does certainly improve the overall quality of the GF bread. Naturally GF starches such as rice, potatoes or tapioca starch, rather than wheat starch, should be used for this purpose. Hydrocolloids are an essential ingredient for GF bread production, since they are able to mimic the visco-elastic properties of gluten to a certain extent. They are also known to reduce staling, improve water binding and improve the overall structure of the bread. Research performed so far indicate xanthan gum and hydroxypropylmethyl- cellulose (HPMC) as the most suitable hydrocolloids for GF bread formulations, but further research is needed to optimise the application of these or other hydrocolloids in GF systems. Protein based ingredients are also essential in the improvement of GF bread, and the most promising are probably the dairy-based ingredients; however, it is essential that only low lactose dairy ingredients are used.
One of the most important ingredients in any GF formulation is water, and therefore it is essential to optimise the water level for every formulation, in order to achieve optimal results. Recently, research has also focused on the application of enzymes to improve the texture of GF bread, but a clear dependency on the raw material has to be taken into consideration. Lactic acid bacteria / GF sourdough are also one possibility to improve GF bread quality, particularly its sensory properties. Even if the research on GF products is still in its infancy, researchers have been able to create products, which are superior to the ones currently on the market, and which coeliac patients might soon be able to see available in the stores (Fig. 4).




VII. AOECS - Association Of European Coeliac Societies
Hertha Deutsch
AOECS-Codex-Delegate, Vienna, Austria
Introduction
AOECS is the umbrella organisation of national coeliac societies in Europe and is an independent non-profit association. AOECS was founded in 1988 and comprises 37 coeliac societies from 33 countries across Europe.
Since 1992 AOECS has the status “Observer” in the world-wide Codex Alimentarius Commission and participated in all sessions of the Commission and some Codex Committees since that time. In various statements AOECS informed about coeliac disease and the high incidence of the gluten intolerant population resulting in the increased awareness of gluten intolerance in the food industry.
AOECS participated very actively in the elaboration and modification of all worldwide Codex Standards and Guidelines for labelling of foods for normal consumption, genetically modified foods and special dietary foods. All details were published in various Proceedings of the Working Group on Prolamin Analysis and Toxicity and are summarised in the paper “20 years AOECS” [1].
With the adoption of the “Codex Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten” in July 2008 [2] and the equivalent “Commission Regulation (EC) No 41/2009” of January 2009 [3] all legal requirements for a save gluten-free diet were established and the most important goal for coeliacs was fulfilled.
However, the threshold to detect gluten in foods is connected with a correct analytical method and a discussion about this subject started again in the Codex Committee on Methods of Analysis and Sampling in March 2010.
Codex Committee on Methods of Analysis and Sampling
The considerations in CCMAS (Codex Committee on Methods of Analysis and Sampling) at the session in March 2010 were reported in the 24th Proceedings of the Working Group on Prolamin Analysis and Toxicity [4].
At the CCMAS session in March 2011 the item “Use of proprietary methods in Codex standards” was discussed at Agenda Nr. 6.
The following AOECS Statement was distributed as a “Conference-Room-Document” to all Codex delegates [5]:
“AOECS (Association Of European Coeliac Societies) is the umbrella organisation of 37 national coeliac societies from 33 countries across Europe and is an independent no profit association. Coeliac societies cooperate with scientists and gastroenterologists to provide people with coeliac disease with information and any help to maintain their gluten-free diet.
We would like to recall our comment on this subject written in para 115 in the report of the 31st session of CCMAS:
“115. The Observer from AOECS recalled that the R5 method is the most accurate method from the scientific point of view for the time being, and that if different methods were allowed, it would create serious problems as to how to handle different results for the same food sample: if one method detects a gluten content higher than 20 mg/kg gluten and another method detects a level below 20 mg/kg, it cannot be determined whether the food can be labelled “gluten free” or not. Moreover, the Observer noted that a method which underestimates the gluten content in foods poses severe health risks for gluten intolerant-consumers.”
In 2010 a further scientific study confirmed the results from previous studies:
considerable differences were demonstrated in the performance of the ELISA kits using differing antibodies. In addition, detection of barley in other ELISA kits is very weak and this issue has not been solved to date. The article is in press and will be available in April 2011, but with the permission of the author Prof. Peter Köhler, Deutsche Forschungsanstalt für Lebensmittelchemie, Freising, Germany, we are permitted to share some of the reported results:
Four different ELISA kits were used. The R5-Gliadin Sandwich ELISA was set to 100 % to prove compatibility with three other ELISA methods: two based on the antigen omega-gliadins, a monoclonal antibody; one based on prolamins, a polyclonal antibody. The signal intensity (%) of the three comparator ELISA kits varies in wheat flour between 52.9 to 78.2, in rye flour between 28.3 to 93.6 and in barley flour between 3.6 to 23.0.
The study also pointed out the differences of the reactivity towards prolamins and glutelins.
Based on this data we urgently ask CCMAS not to endorse other methods of gluten detection to avoid possible severe health risks for people with coeliac disease because of underestimating the prolamin content in foods.
AOECS welcomes any further research:
- to develop new methods
- or improve existing methods
- or develop a new antibody showing reactivity towards all of the potentially toxic sequences.
However, before any other method is adopted by CCMAS, the compatibility of methods should be guaranteed and proven by ring tests.
We would like to recall that the labelling “gluten-free” is connected with the analytical method of gluten detection. Differing measurements of gluten levels in the same food sample caused by using different methods will create severe problems for international food trade and also for national food control institutes. We noted that concerns have been raised regarding the availability of the reference material (PWG Gliadin) and would like to inform CCMAS that enough reference material is available and can be produced again at any time.
Finally AOECS would like to inform CCMAS that an extended ring trial comparing various methods will start in 2011.”
This item was extensively discussed at the IAM/MoniQA Workshop, which was taken place the day before the beginning of the CCMAS session and networking with some delegates was very fruitful.
The CCMAS did not change the type of the R5-method at this session and agreed to initiate new work on the development of provisions for proprietary methods in the Procedural Manual. An electronic working group would define the term “proprietary method” and prepare a draft version of the criteria to be included in the Procedual Manual [6].
European Commission
In June 2011 we were informed about the work of the European Commission revising the legislation of dietetic foods and in doing so repeal the Commission Regulation (EC) 41/2009 “concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten”. The European Commission intended to regulate the requirements for 41/2009 under Regulation (EC) 1924/2006 which sets requirements on nutrition and health claims. Because of the lobbying from coeliac societies at national level and AOECS on international level the Regulation 41/2009 was not repealed in 2011 and it looks like AOECS was successful to prevent that the gluten issue will be regulated in the Regulation (EC) 1924/2006. AOECS and its members will participate very actively in further considerations with authorities.
References
1. Deutsch H. “20 years AOECS”, Proceedings of the 23rd Meeting, Working Group on Prolamin Analysis and Toxicity, 25-27 September 2008, Barcelona, Spain, pp. 125-137. 2. CODEX STAN 118 - 1979 CODEX STANDARD FOR FOODS FOR SPECIAL DIETARY USE FOR PERSONS INTOLERANT TO GLUTEN, adopted in 1979; amended 1983; revised 2008.
3. COMMISSION REGULATION (EC) No 41/2009 of 20 January 2009 concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten.
4. Deutsch H. “AOECS Association of European Coeliac Societies”, Proceedings of the 24th Meeting, Working Group on Prolamin Analysis and Toxicity, 30 September – 2 October 2010, Ancona, Italy, pp. 101-105.
5. CRD 4 (Comments of AOECS and ISO) published at the 32th session of the Codex Committee on Methods of Analysis and Sampling, Budapest, Hungary, 7-11 March 2011.
6. Report of the 32th session of the Codex Committee on Methods of Analysis and Sampling, Budapest, Hungary, 7-11 March 2011, page 7, para 73-78.
VIII. Coeliac disease and evidence-based
regulations of gluten-free food.
The Prolamin Working Group 1985-2011
Coeliac disease and evidence-based regulations of gluten-free food
The Prolamin Working Group 1985-2011
Martin Stern
University Children's Hospital, Tuebingen, Germany
The international Working Group on Prolamin Analysis and Toxicity (WGPAT, PWG) was founded in 1985 by Wim Hekkens, University of Leiden, The Netherlands, to coordinate research on laboratory gluten analysis in food and on clinical evaluation of effects ("toxicity") of food in coeliac patients. This group (list of members see under table of contents) comprises physicians, chemists, nutritionists, food scientists from different countries, and has successfully cooperated with the starch-producing industry, with manufacturers of gluten-free products, with manufacturers of test systems for gluten analysis, with national and international coeliac societies and with organisations such as the Codex Alimentarius Committee on Nutrition and Food for Special Dietary Uses (CCNFSDU). In the years of 1991 to 2011, Martin Stern, University of Tuebingen, Germany, has been chairman to the group. In 1999, the group gained non-governmental organisational status as an observer to Codex Alimentarius [1].
By a dual approach, laboratory methods of gluten analysis and clinical research into gluten sensitivity have been tackled [2]. The wide clinical spectrum and new insight into the autoimmune pathophysiology of coeliac disease have been the background of the group´s work towards improved evidence-based regulations of gluten-free food [3,4]. Conventional earlier ELISA methods for gluten analysis were found not adequately sensitive and reliable. This was changed by the introduction of the R5 sandwich and later competitive ELISA systems by Enrique Méndez and his coworkers [5-7]. The monoclonal R5 antibody, which forms the basis of this new ELISA system, is directed towards the gluten peptide QQPFP in gliadins, hordeins and secalins. Immunochemical work was accompanied by mass spectrometry, HPLC, SDS-PAGE and capillary electrophoresis. In 2006, the R5 ELISA method was endorsed as a type 1 method by the Codex Committee on Methods of Analysis and Sampling (CCMAS).
WGPAT has worked towards a gliadin standard and has produced PWG gliadin as a basis for further standardisation [8,9]. This material is available in 100 mg batches from WGPAT (peter.koehler@tum.de).
Clinical work was conducted by members of the group first on oats and later on a potential "safe threshold" for gluten contamination and gluten-free products [10,11]. Together with new consumption data [12] these three scientific steps (PWG gliadin, R5 ELISA, new clinical information) led the Codex Alimentarius Commission to adopt the draft-revised codex standard for foods for special dietary use for persons intolerant to gluten finally in 2008 [13]. The limits of 20 mg/kg gluten in food glutenfree by nature and of 100 mg/kg in food especially processed to reduce gluten content have been set and since used successfully.
WGPAT has produced annual book reports of its 25 meetings. The group has opened a homepage: www.wgpat.com.ar, where additional information is to be found on group meetings, discussions and on handling of PWG gliadin.
Normally, coeliac disease is responsive to treatment by a gluten-free diet [14], particularly in children and adolescents, less so in adult patients where symptoms might persist and where even a condition known as "refractory coeliac disease" exists [15]. Thus, open questions remain for future work of WGPAT. Not much is known about long-term sensitivity data. Consumption studies might have settled disputes about national disparities in gluten intake, but still a safety factor of evidence-based regulations of gluten-free food might have to be considered. The question of glutenin toxicity is still not finally solved. The acceptance of pure oats for coeliac patients is increasing, long-term data are missing.
Aspects of morbidity and quality of life of coeliac patients on a gluten-free diat merit further work. New gluten technology is adding positive potential on one hand and potential sources of hidden gluten on the other. A new standard, new methods and novel therapies appear at the horizon [4,16]. Questions of non-coeliac gluten sensitivity [17] and also on non-gluten wheat sensitivity (wheat amylase trypsin inhibitor toxicity) are extending the perspective. WGPAT under its new chairman Peter Koehler, Freising, Germany, continues to offer its help to advance science and practice as well as evidence-based food regulations in the dietary therapy of coeliac disease.
Acknowledgement
The author gratefully acknowledges the long-standing secretarial help by Dr. Gisa Briese-Neumann.
References
1. Stern M, Ciclitira PJ, van Eckert R, et al. Analysis and clinical effects of gluten in coeliac disease. Eur J Gastroenterol Hepatol 2001; 13: 741-747.
2. Wieser H, Koehler, P. The biochemical basis of celiac disease. Cereal Chem 2008; 85: 1-13.
3. Tack GJ, Verbeek WHM, Schreurs MWJ, et al. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol 2010; doi:10.1038/nrgastro.2010.23
4. Schuppan D, Junker Y, Barisani D. Celiac Disease: From pathogenesis to novel therapies. Gastroenterol 2009; 137: 1912-1933.
5. Valdés I, García E, Llorente M, et al. Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur J Gastroenterol Hepatol 2003; 15: 465-474.
6. Méndez E, Vela C, Immer U, et al. Report of a collaborative trial to investigate performance of the R5 enzyme-linked immunoassay to determine gliadin and gluten-free food. Eur J Gastroenterol Hepatol 2005; 17: 1053-1063.
7. Hernando A, Mujico JR, Mena MC, et al. Measurement of wheat gluten and barley hordeins in contaminated oats from Europe, the United States and Canada by Sandwich R5 ELISA. Eur J Gastroenterol Hepatol 2008; 20: 545-554.
8. Van Eckert R, Berghofer E, Ciclitira PJ, et al. Towards a new gliadin reference material - isolation and characterisation. J Cereal Sci 2006; 43: 331-341.
9. Van Eckert R, Bond J, Rawson P, et al. Reactivity of gluten detecting monoclonal antibodies to a gliadin reference material. J Cereal Sci 2010; 51: 198-204.
10. Collin P, Thorell L, Kaukinen K, et al. The safe threshold for gluten contamination in gluten-free products: can trace amounts be accepted in the treatment of coeliac disease? Aliment Pharmacol Ther 2004; 29: 1277-1283.
11. Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebocontrolled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007; 85: 160-166.
12. Gibert A, Espadaler M, Angel Calena M, et al. Consumption of gluten-free products: should the threshold value for trace amounts of gluten be at 20, 100 or 200 ppm? Eur J Gastroenterol Hepatol 2006; 18: 1187-1195.
13. Draft revised codex standard for foods for special dietary use for persons intolerant to gluten. Alinorm 08/31/26, Appendix III, Codex Alimentarius.
14. Stern M. Current therapy. In: Fasano A, Troncone R, Branski D (eds): Frontiers in celiac disease. Pediatr Adolesc Med Basel, Karger, 2008; 12: 114-122.
15. Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut 2010; 59: 547-557.
16. Sollid LM, Khosla C. Novel therapies for coeliac disease. J Internal Med 2011; 269: 604-613.
17. Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebocontrolled trial. Am J Gastroenterol 2011; 106: 508-514.
IX. Perspectives and action plan
Perspectives and action plan
Peter Koehler
German Research Centre for Food Chemistry, Freising, Germany
The Prolamin Working Group executive meeting and joint discussion held on October 1, 2011 led to the decisions outlined below.
Action plan
I. Analytical
PWG gliadin is available in 100 mg batches. Distribution by Prof. Martin Stern is discontinued in 2012. Prof. Peter Koehler will take over the responsibility for this reference material from April 1, 2012 (Peter.Koehler@tum.de).
The group agrees that ideas for a new reference material for gluten analysis should be collected, which will be discussed during the next meeting.
R5 ELISA for gluten quantification using PWG gliadin as a reference material should be accepted as approved method with different organisations.
Collaborative studies with ELISA kits containing new antibodies such as G12 and 20 should be organised by the PWG in the future.
II. Clinical
Studies on mechanisms of innate immunity and gluten sensitivity are a focus in the next years.
New diagnostic antibody assays are still an important topic.
III. Publication and policy
Prof. Detlef Schuppan (Mainz, Germany) and Dr. Luud Gilissen (Wageningen, The Netherlands) were introduced as new group members. Dr. Frederik Janssen (Zutphen, The Netherlands) and Dr. Katri Kaukinen (Tampere, Finland) have left the group.
The PWG homepage will be re-constructed and re-launched. The group agrees that the availability of online information will increasingly be important in the future. Some sections need to be re-written.
This printed, citable book (print run: 500 copies with ISBN number) was made possible by funding of Dr. SCHÄR GmbH/Srl, (Burgstall, BZ, Italy) and by the production team of Deutsche Forschungsanstalt für Lebensmittelchemie (Freising, Germany). It will be distributed among leaders of opinion in gluten analysis and clinical medicine.
|
|
List of Participants
GROUP MEMBERS
Prof. Dr. Carlo Catassi
Università politecnica delle marche Facoltà di Medicina e Chirurgia
Istituto di Clinica Pediatrica
Via Corridoni 11 - 60123 ANCONA, IT ALY
Phone +39 349 2235 447 | Tele fax +39 071 36281
Email: catassi@tin.it
Prof. Dr. Fernando G. Chirdo
Laboratorio de Investigación en el Sistema Inmune (LISIN)
Facultad de Ciencias Exactas Universidad Nacional de La Plata cc 711
(1900) LA PLATA, ARGENTINA
Phone: +54 221 421 0 497 | 423 0 121 | 423 5 333 (Int 45)
Tele fax +54 221 422 6947
Email: fchirdo@biol.unlp.edu.ar
Prof. Paul J. Ciclitira
King's College London (Division of Diabetes and Nutritional Sciences) The Rayne Institute (KCL). St Thomas' Hospital Westminster Bridge Road. LONDON SE1 7EH, UK/ENGLAND
Phone: +44 207 620 2597 | 207 188 2494 | Telefax +44 207 261 0667
Email: mila.labar_weintrop@kcl.ac.uk (secretary)
Email: paul.ciclitira@kcl.ac.uk
Prof. Conleth Feighery, MD
(not at tend ing)
University of Dublin Department of Immunology St. James's Hospital James's Street DUB LIN 8, IRELAND
Phone: +353 1 896 3432 | Tele fax +353 1 4545-609
Email: con.feighery@tcd.ie
Dr. Luud Gilissen
Plant Research International (PRI) Wageningen University
Droevendaalsesteeg 1 6708 PB WAGENINGEN, THE NETHERLANDS
Phone: +31 317-480983 | Fax: +31 317-418094
Email: luud.gilissen@wur.nl
Prof. Dr. Peter Köhler
Deut sche Forschungsanstalt für Lebensmittelchemie
Lise-Meitner-Straße : +34 - 85354
FREISING, GER MANY
Phone: +49 81 61 71 29 28 | Tele fax +49 81 61 71 29 70
Email: peter.koehler@tum.de
Prof. Dr. Frits Koning
Leiden Univer sity Medical Center, E3-Q
Department of Immunohaematology and Bloodbank Albinusdreef 2
2333 ZA LEIDEN, THE NETHERLANDS
Phone: +31 71 5266673 | Tele fax +31 71 5265267
Email: fkoning@lumc.nl
Prof. Dr. Thomas Mothes
Universitätsklinikum Leip zig A. ö. R.
Institut für Laboratoriumsmedizin, Klinische Chemie und Molekulare Diagnostik - Liebigstraße : 27 - 04103
LEIPZIG, GERMANY
Phone: +49 341 97 22251 | Tele fax +49 341 97 22329
Email: mothes@medizin.uni-leipzig.de
Prof. Dr. Dr. Detlef Schuppan
I. Medizinische Klinik und Poliklinik Universitätsmedizin der Johannes
Gutenberg-Universität Mainz Langenbeckstr. 1,55131 MAINZ, GERMANY
Phone +49 6131-177355/ 177356/177104 - Fax +49 6131-177357
Email: detlef.schuppan@unimedizinmainz.de
Prof. Dr. Martin Stern
Universitätsklinik für Kinder- und Jugendmedizin Hoppe-Seyler-Straße 1
72076 TÜBINGEN, GER MANY
Phone: +49 7071 29 83781 | Tele fax +49 7071 29 5477
Email: martin.stern@med.uni-tuebingen.de
Prof. Dr. Riccardo Troncone (not attending)
Department of Pediatrics and European Laboratory for the Investigation of Food-induced Diseases University of Naples “Federico II”- via Pansini, 5 - 80131 NAPLES, ITALY
Phone: +39 081 7463383 | Telefax +39 081 5469811
Email: troncone@unina.it
Dr. Renate van Eckert
Victo ria Univer sity of Wellington
Centre of Biodiscovery and School of Biological Sciences
P.O. Box 600 WELLINGTON, NEW ZEA LAND
Phone: +64 4 463 6092 | Tele fax +64 4 463 5331
Email: renate.vaneckert@gmail.com
HOST
Sofia Beisel
Deutsche Zöliakie Gesellschaft e.V. Kupferstr. 36
70565 STUTTGART, GERMANY
Phone: +49 711 459981 15 | Telefax +49 711 459981 50
Email: sofia.beisel@dzg-online.de
Judith Suck
DZG e.V. Kupferstr. 36 - 70565 STUTTGART, GERMANY
Phone: +49 711 459981 13 | Telefax +49 711 459981 50
Email: judith.suck@dzg-online.de
Andreas Abbrecht
Deutsche Zöliakie Gesellschaft e.V. Kupferstr. 36
70565 STUTTGART, GERMANY
Phone: +49 711 459981 0 | Telefax +49 711 459981 50
Email: andreas.abbrecht@dzg-online.de
INVITED SPEAKERS
Prof. Dr. Elke K. Arendt
Dept of Food & Nutritional Sciences University College Cork
CORK, IRELAND
Phone: +353 21 490 3000 | Telefax +353 21 490 2064
Email: E.Arendt@ucc.ie
Dr. Jussi Loponen
R&D Research Technologist Fazer Group Fazerintie 6, 01230 Vantaa
PO Box 4 - FI-00941 HELSINKI, FINLAND
Phone-mobil: +358 40 732 9772
Email: jussi.loponen@fazer.com
GUESTS
Dr. Markus Brandt
Ernst Böcker GmbH & Co. KG - Ringstraße 55-57
32427 MINDEN, GERMANY
Phone: +49 571 8379943 | Telefax +49 571 83799943
Email: markus.brandt@sauerteig.de
Laia Cabré
Celíacs de Catalunya Balmes 109, Pral. 2a - 08008 BARCELONA, SPAIN
Phone +93 412 1789 - Telefax +93 487 5858
Email: laia@celiacscatalunya.orf
Isabel Maria Comino Montilla
Facultad de Farmacia, Universidad De Sevilla, C/ Professor Garcia
Dpto Microbiologia y Parasitologia - González n°2, 41012 SEVILLA
Phone: +34 95455 6453
Email: icomino@us.es
Hertha Deutsch
AOECS - Association of European Coeliac Societies-Codex and Regulatory Affairs - Anton Baumgartner Straße 44/C5/2302
1230 VIENNA, AUSTRIA
Phone: +43 1 66 71887 | Telefax +43 1 66 71887
Email: hertha.deutsch@utanet.at
Dr. Clyde Don
Foodphysica - Vogelwikke 12 - 6665 HP DRIEL - THE NETHERLANDS
Phone: +31 622 543 047
Email: clyde.don@foodphysica.com
Margaret Dunne
Department of Immunology Trinity College and St. James´s Hospital Dublin - 228 Cooley Road, Drimnagh - DUBLIN 12, IRELAND
Phone: +353 857 355787
Email: Margaret.a.dunne@gmail.com
Dr. Richard J. Fielder
ROMER LABS UK Limited Building 5325 - North Wales Buisness Park
ABERGELE LL22 8LJ - UK/ENGLAND
Phone: +44 1745 826016 | Telefax +44 1745 827150
Email: richard.fielder@romerlabs.com
Dr. Christian Gößwein
R-Biopharm AG An der neuen Bergstraße 17 - 64297 DARMSTADT, GERMANY
Phone: +49 6151 81 02578 | Telefax +49 6151 81 02 20
Email: c.goesswein@r-biopharm.de
Marta Gomez Pes
Celíacs de Catalunya Balmes 109, Pral. 2a - 08008 BARCELONA, SPAIN
Phone: +34 93 412 1789 | Telefax +34 93 487 58 58
Email: marta@celiacscatalunya.org
Gertrud Granel
Fachverband der Stärke-Industrie e.V. Königstraße 57
56115 BONN, GERMANY
Phone: +49 30 8871 3398-15 | Telefax +49 30 8871 3398-19
Email: g.granel@verbaende-jess.de
Elisabeth Hammer
Product Manager Romer Labs Division Holding GmbH Technopark 1
3430 TULLN, AUSTRIA
Phone: +43 2272 615 33 122 | Telefax +43 2272 615 33 111
Email: elisabeth.hammer@romerlabs.com
Dr. Ulrike Immer
R-Biopharm AG An der neuen Bergstraße 17
64297 DARMSTADT, GERMANY
Phone: +49 6151 6802 38 | Telefax +49 6151 6802 20
Email: u.immer@r-biopharm.de
Dr. Päivi Kanerva
Department of Food and Evironmental Sciences - P.O. Box 66
Agnes Sjöbergin katu 2 - 00014 UNIVERSITY OF HELSINKI
HELSINKI, FINLAND
Phone: +358 9 191 58236 | Telefax +358 9 191 58460
Email: paivi.kanerva@helsinki.fi
Verena Knorr
Dt. Forschungsanstalt für Lebensmittelchemie Lise-Meitner-Straße 34
85354 FREISING, GERMANY
Phone: +49 08161 71 2926 | Telefax +49 08161 71 2970
Email: Verena.Knorr@lrz.tum.de
Dr. Götz Kröner
Hermann Kröner GmbH Im Bocketal 21 - 49479 IBBENBÜREN, GERMANY
Phone: +49 05451-9447-0 | Fax +05451-9447-39
Email: kroener@kroener-staerke.de
Dr. Astrid Kruizinga, M.Sc.
TNO Quality of Life Utrechtseweg 48 - P.O. Box 360 - 3700 AJ ZEIST
THE NETHERLANDS
Phone: +31 30 694 47 70 | Telefax +31 30 694 40 70
Email: astrid.kruizinga@tno.nl
Stella Lindeke
R-Biopharm AG - An der neuen Bergstaße 17
64297 DARMSTADT, GERMANY
Phone: +49 6151 8102 92 | Telefax +49 6151 8102 40
Email: s.lindeke@r-biopharm.de
Iris Lovric
Deutsche Forschungsanstalt für Lebensmittelchemie Lise-Meitner-Straße 34 - 85354 FREISING, GERMANY
Phone: +49 08161 71 2926 | Telefax +49 08161 71 2970
Email: Iris.Lovric@lrz.tum.de
Gabriela Ratz
Deutsche. Forschungsanstalt für Lebensmittelchemie Lise-Meitner-Straße 34 - 85354 FREISING, GERMANY
Phone: +49 08161 71 2941 | Telefax +49 08161 71 2970
Email: Gabriela.Ratz@lrz.tum.de
Mareike Richter
R-Biopharm AG An der neuen Bergstaße 17
64297 DARMSTADT, GERMANY
Phone: +49 6151 8102 875 | Telefax +49 6151 8102 40
Email: m.richter@r-biopharm.de
Cristina Romero
INGENASA Hnos. García Noblejas No. 39 - 8a Planta
28037 MADRID, SPAIN
Phone: +34 91 3680501 | Telefax +34 91 4087598
Email: cromero@ingenasa.es
Theresa Schwalb
Deutsche Forschungsanstalt für Lebensmittelchemie Lise-Meitner-Straße 34 - 85354 FREISING, GERMANY
Phone: +49 08161 9868430 | Telefax +40 08161 71 2970
Email: Theresa.Schwalb@lrz.tum.de
Juan Ignacio Serrano-Vela
Asociación de Celíacos de Madrid - (ACM) - C/Lanuza, 19-Local, Izquierdo - 28028 MADRID, SPAIN
Phone: +34 91 7130147 | Telefax + 34 91 7258059
Email: nachoserrano@celiacosmadrid.org
Ylva Sjögren Bolin
National Food Administration Research & Development P.O. Box 622
SE-751 26 UPPSALA, SCHWEDEN
Phone: +46 18 171416 | Telefax +46 18 105848
Email: ylva.sjogren@slv.se
Dr. Franceso Valitutti
Associazione Italiana Celiachia - Via Caffaro 68R
16125 GENOVA, ITALY
Phone: +39 346 9532264
Email: fvalitutti@celiachia.it
Maren Wiese
Hermann Kröner GmbH - Postfach 1354
49479 IBBENBÜREN, GERMANY
Phone: +49 5451 9447 12 | Telefax +49 5451 9447 39
Email: wiese@kroener-staerke.de
Victor Zevallos
Translationale Medizin I, Med. - Klinik & Poliklinik - Langenbeckstraße 1
55131 MAINZ, GERMANY
Phone: +49 6131 17 9783 | Telefax + 49 6131 17 9988
Email: zevallos@uni-mainz.de
Impressum
Proceedings of the 25th Meeting
WORKING GROUP
on PROLAMIN ANALYSIS and TOXICITY
September 29 – October 2, 2011
Fellbach, Germany
This work including all parts is subject to copyright. All rights are reserved and any utilisation is only permitted under the provisions of the German Copyright Law.
Permissions for use must always be obtained from the publisher. This is in particular valid for reproduction, translation, conversion to microfilm and for storage or processing in electronic systems.
Scientific Organisation
Prof. Dr. Martin Stern
Universitätsklinik für Kinder- und Jugendmedizin
Hoppe-Seyler-Straße 1, 72076 TÜBINGEN, GERMANY
Phone: +49 7071 2983781 | Telefax +49 7071 295477
Email: martin.stern@med.uni-tuebingen.de
Host
Deutsche Zöliakiegesellschaft e.V.
Kupferstraße 36, 70565 STUTTGART, GERMANY
Phone: +49 711 459981-15 | Telefax: +49 711 459981-50
Email: sofia.beisel@dzg-online.de
Cover picture
Thomas Mothes
© Verlag Deutsche Forschungsanstalt für Lebensmittelchemie (DFA)
Lise-Meitner-Strasse 34, 85354 Freising
Phone: +49 8161 712928
www.dfal.de
ISBN: 978-3-938896-54-9
|


